Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling
- PMID: 19369419
- PMCID: PMC2688552
- DOI: 10.1091/mbc.e08-11-1102
Eps15 homology domain 1-associated tubules contain phosphatidylinositol-4-phosphate and phosphatidylinositol-(4,5)-bisphosphate and are required for efficient recycling
Abstract
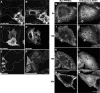
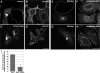
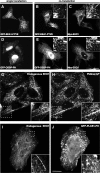
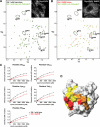
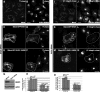
The C-terminal Eps15 homology domain (EHD) 1/receptor-mediated endocytosis-1 protein regulates recycling of proteins and lipids from the recycling compartment to the plasma membrane. Recent studies have provided insight into the mode by which EHD1-associated tubular membranes are generated and the mechanisms by which EHD1 functions. Despite these advances, the physiological function of these striking EHD1-associated tubular membranes remains unknown. Nuclear magnetic resonance spectroscopy demonstrated that the Eps15 homology (EH) domain of EHD1 binds to phosphoinositides, including phosphatidylinositol-4-phosphate. Herein, we identify phosphatidylinositol-4-phosphate as an essential component of EHD1-associated tubules in vivo. Indeed, an EHD1 EH domain mutant (K483E) that associates exclusively with punctate membranes displayed decreased binding to phosphatidylinositol-4-phosphate and other phosphoinositides. Moreover, we provide evidence that although the tubular membranes to which EHD1 associates may be stabilized and/or enhanced by EHD1 expression, these membranes are, at least in part, pre-existing structures. Finally, to underscore the function of EHD1-containing tubules in vivo, we used a small interfering RNA (siRNA)/rescue assay. On transfection, wild-type, tubule-associated, siRNA-resistant EHD1 rescued transferrin and beta1 integrin recycling defects observed in EHD1-depleted cells, whereas expression of the EHD1 K483E mutant did not. We propose that phosphatidylinositol-4-phosphate is an essential component of EHD1-associated tubules that also contain phosphatidylinositol-(4,5)-bisphosphate and that these structures are required for efficient recycling to the plasma membrane.
Figures

References
-
- Balla A., Tuymetova G., Tsiomenko A., Varnai P., Balla T. A plasma membrane pool of phosphatidylinositol 4-phosphate is generated by phosphatidylinositol 4-kinase type-III alpha: studies with the PH domains of the oxysterol binding protein and FAPP1. Mol. Biol. Cell. 2005;16:1282–1295. - PMC - PubMed
-
- Blume J. J., Halbach A., Behrendt D., Paulsson M., Plomann M. EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp. Cell Res. 2007;313:219–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

