Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects
- PMID: 19369543
- PMCID: PMC2721059
- DOI: 10.1523/JNEUROSCI.0325-09.2009
Connexin32 mutations cause loss of function in Schwann cells and oligodendrocytes leading to PNS and CNS myelination defects
Abstract
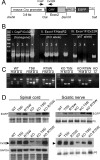
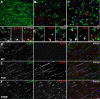
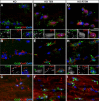
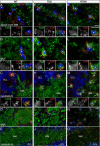
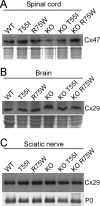
The gap junction (GJ) protein connexin32 (Cx32) is expressed by myelinating Schwann cells and oligodendrocytes and is mutated in X-linked Charcot-Marie-Tooth disease. In addition to a demyelinating peripheral neuropathy, some Cx32 mutants are associated with transient or chronic CNS phenotypes. To investigate the molecular basis of these phenotypes, we generated transgenic mice expressing the T55I or the R75W mutation and an IRES-EGFP, driven by the mouse Cnp promoter. The transgene was expressed in oligodendrocytes throughout the CNS and in Schwann cells. Both the T55I and the R75W mutants were localized in the perinuclear cytoplasm, did not form GJ plaques, and did not alter the expression or localization of two other oligodendrocytic GJ proteins, Cx47 and Cx29, or the expression of Cx29 in Schwann cells. On wild type background, the expression of endogenous mCx32 was unaffected by the T55I mutant, but was partly impaired by R75W. Transgenic mice with the R75W mutation and all mutant animals with Gjb1-null background developed a progressive demyelinating peripheral neuropathy along with CNS myelination defects. These findings suggest that Cx32 mutations result in loss of function in myelinated cells without trans-dominant effects on other GJ proteins. Loss of Cx32 function alone in the CNS causes myelination defects.
Figures

Similar articles
-
Connexin32-null mice develop demyelinating peripheral neuropathy.Glia. 1998 Sep;24(1):8-20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. Glia. 1998. PMID: 9700485
-
Intrathecal gene therapy in mouse models expressing CMT1X mutations.Hum Mol Genet. 2018 Apr 15;27(8):1460-1473. doi: 10.1093/hmg/ddy056. Hum Mol Genet. 2018. PMID: 29462293
-
Prenylation-defective human connexin32 mutants are normally localized and function equivalently to wild-type connexin32 in myelinating Schwann cells.J Neurosci. 2005 Aug 3;25(31):7111-20. doi: 10.1523/JNEUROSCI.1319-05.2005. J Neurosci. 2005. PMID: 16079393 Free PMC article.
-
Gap junction disorders of myelinating cells.Rev Neurosci. 2010;21(5):397-419. doi: 10.1515/revneuro.2010.21.5.397. Rev Neurosci. 2010. PMID: 21280457 Review.
-
Molecular mechanisms of gap junction mutations in myelinating cells.Histol Histopathol. 2010 Sep;25(9):1191-206. doi: 10.14670/HH-25.1191. Histol Histopathol. 2010. PMID: 20607661 Review.
Cited by
-
Cross-Sectional Study in a Large Cohort of Chinese Patients With GJB1 Gene Mutations.Front Neurol. 2020 Jul 31;11:690. doi: 10.3389/fneur.2020.00690. eCollection 2020. Front Neurol. 2020. PMID: 32903794 Free PMC article.
-
X-linked Charcot Marie Tooth mutations alter CO2 sensitivity of connexin32 hemichannels.Front Cell Neurosci. 2023 Dec 21;17:1330983. doi: 10.3389/fncel.2023.1330983. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38188670 Free PMC article.
-
Emerging Therapies for Charcot-Marie-Tooth Inherited Neuropathies.Int J Mol Sci. 2021 Jun 3;22(11):6048. doi: 10.3390/ijms22116048. Int J Mol Sci. 2021. PMID: 34205075 Free PMC article. Review.
-
Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans.PLoS Genet. 2011 Jul;7(7):e1002146. doi: 10.1371/journal.pgen.1002146. Epub 2011 Jul 7. PLoS Genet. 2011. PMID: 21750683 Free PMC article.
-
Functional heterotypic interactions between astrocyte and oligodendrocyte connexins.Glia. 2011 Jan;59(1):26-34. doi: 10.1002/glia.21073. Glia. 2011. PMID: 21046554 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
