Ensheathing glia function as phagocytes in the adult Drosophila brain
- PMID: 19369546
- PMCID: PMC2674269
- DOI: 10.1523/JNEUROSCI.5951-08.2009
Ensheathing glia function as phagocytes in the adult Drosophila brain
Abstract
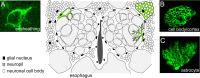
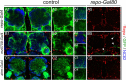
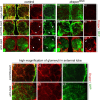
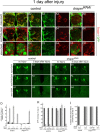
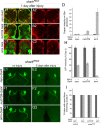
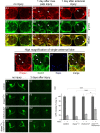
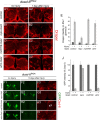
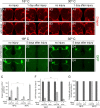
The mammalian brain contains many subtypes of glia that vary in their morphologies, gene expression profiles, and functional roles; however, the functional diversity of glia in the adult Drosophila brain remains poorly defined. Here we define the diversity of glial subtypes that exist in the adult Drosophila brain, show they bear striking similarity to mammalian brain glia, and identify the major phagocytic cell type responsible for engulfing degenerating axons after acute axotomy. We find that neuropil regions contain two different populations of glia: ensheathing glia and astrocytes. Ensheathing glia enwrap major structures in the adult brain, but are not closely associated with synapses. Interestingly, we find these glia uniquely express key components of the glial phagocytic machinery (e.g., the engulfment receptor Draper, and dCed-6), respond morphologically to axon injury, and autonomously require components of the Draper signaling pathway for successful clearance of degenerating axons from the injured brain. Astrocytic glia, in contrast, do not express Draper or dCed-6, fail to respond morphologically to axon injury, and appear to play no role in clearance of degenerating axons from the brain. However, astrocytic glia are closely associated with synaptic regions in neuropil, and express excitatory amino acid transporters, which are presumably required for the clearance of excess neurotransmitters at the synaptic cleft. Together these results argue that ensheathing glia and astrocytes are preprogrammed cell types in the adult Drosophila brain, with ensheathing glia acting as phagocytes after axotomy, and astrocytes potentially modulating synapse formation and signaling.
Figures
References
-
- Auld VJ, Fetter RD, Broadie K, Goodman CS. Gliotactin, a novel transmembrane protein on peripheral glia, is required to form the blood-nerve barrier in Drosophila. Cell. 1995;81:757–767. - PubMed
-
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–867. - PubMed
-
- Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. - PubMed
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
