Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex
- PMID: 19369560
- PMCID: PMC6665345
- DOI: 10.1523/JNEUROSCI.0332-09.2009
Linking the input to the output: new sets of neurons complement the polarization vision network in the locust central complex
Abstract
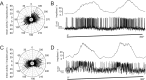
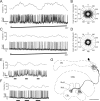
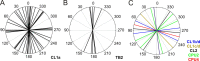
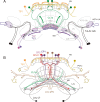
Polarized light is a key feature of the blue sky, used by many animals as a sensory cue for compass navigation. Like other insects, locusts perceive the E-vector orientation of polarized light with a specialized region of their compound eye, the dorsal rim area. Neurons in the brain relay this information through several processing stages to the central complex. The central complex has a modular neuroarchitecture, composed of vertical columns and horizontal layers. Several types of central-complex neurons respond to dorsally presented, rotating E-vectors with tonic modulation of their firing frequency. These neurons were found at the input stage of the central complex, as well as near the proposed output stage, where neurons are tuned to form a compass-like representation of E-vector orientations underlying the columnar organization of the central complex. To identify neurons suited to link input and output elements, we recorded intracellularly from 45 neurons of the central complex. We report several novel types of polarization-sensitive neurons. One of these is suited to fill the gap between input and output stages of the central-complex polarization vision network. Three types of neurons were sensitive to polarized light in only 50% of experiments suggesting that they are recruited to the network depending on behavioral context. Finally, we identified two types of neurons suited to transfer information toward thoracic motor circuits. The data underscore the key role of two subunits of the central complex, the lower division of the central body and the protocerebral bridge, in sky compass orientation.
Figures






Similar articles
-
Transformation of polarized light information in the central complex of the locust.J Neurosci. 2009 Sep 23;29(38):11783-93. doi: 10.1523/JNEUROSCI.1870-09.2009. J Neurosci. 2009. PMID: 19776265 Free PMC article.
-
Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light.J Neurosci. 2002 Feb 1;22(3):1114-25. doi: 10.1523/JNEUROSCI.22-03-01114.2002. J Neurosci. 2002. PMID: 11826140 Free PMC article.
-
Polarization-sensitive and light-sensitive neurons in two parallel pathways passing through the anterior optic tubercle in the locust brain.J Neurophysiol. 2005 Dec;94(6):3903-15. doi: 10.1152/jn.00276.2005. Epub 2005 Jul 27. J Neurophysiol. 2005. PMID: 16049147
-
In search of the sky compass in the insect brain.Naturwissenschaften. 2004 May;91(5):199-208. doi: 10.1007/s00114-004-0525-9. Epub 2004 Apr 20. Naturwissenschaften. 2004. PMID: 15146265 Review.
-
Central neural coding of sky polarization in insects.Philos Trans R Soc Lond B Biol Sci. 2011 Mar 12;366(1565):680-7. doi: 10.1098/rstb.2010.0199. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21282171 Free PMC article. Review.
Cited by
-
The Locust Standard Brain: A 3D Standard of the Central Complex as a Platform for Neural Network Analysis.Front Syst Neurosci. 2010 Feb 3;3:21. doi: 10.3389/neuro.06.021.2009. eCollection 2009. Front Syst Neurosci. 2010. PMID: 20161763 Free PMC article.
-
Physiological Signatures of Changes in Honeybee's Central Complex During Wing Flapping.J Insect Sci. 2022 Sep 1;22(5):10. doi: 10.1093/jisesa/ieac060. J Insect Sci. 2022. PMID: 36222481 Free PMC article.
-
In search of behavioral and brain processes involved in honey bee dance communication.Front Behav Neurosci. 2023 Jun 29;17:1140657. doi: 10.3389/fnbeh.2023.1140657. eCollection 2023. Front Behav Neurosci. 2023. PMID: 37456809 Free PMC article. Review.
-
The Topographical Mapping in Drosophila Central Complex Network and Its Signal Routing.Front Neuroinform. 2017 Apr 10;11:26. doi: 10.3389/fninf.2017.00026. eCollection 2017. Front Neuroinform. 2017. PMID: 28443014 Free PMC article.
-
A Neurocomputational Model of Goal-Directed Navigation in Insect-Inspired Artificial Agents.Front Neurorobot. 2017 Apr 12;11:20. doi: 10.3389/fnbot.2017.00020. eCollection 2017. Front Neurorobot. 2017. PMID: 28446872 Free PMC article.
References
-
- Batschelet E. Circular statistics in biology. London: Academic; 1981.
-
- Clements AN, May TE. Studies on locust neuromuscular physiology in relation to glutamic acid. J Exp Biol. 1974;60:673–705. - PubMed
-
- Eggers A, Gewecke M. The dorsal rim area of the compound eye and polarization vision in the desert locust (Schistocerca gregaria) In: Wiese K, Gribakin FG, Popov AV, Renninger G, editors. Sensory systems of arthropods. Basel: Birkhäuser; 1993. pp. 101–109.
-
- Hanesch U, Fischbach KF, Heisenberg M. Neuronal architecture of the central complex in Drosophila melanogaster. Cell Tissue Res. 1989;257:343–366.
-
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science. 2007;315:995–997. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
