Substance P mediates excitatory interactions between striatal projection neurons
- PMID: 19369564
- PMCID: PMC6665341
- DOI: 10.1523/JNEUROSCI.6020-08.2009
Substance P mediates excitatory interactions between striatal projection neurons
Abstract
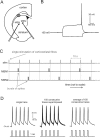
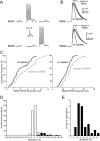
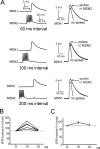
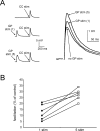
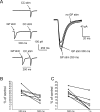
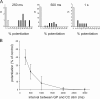
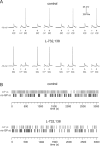
The striatum is the largest nucleus of the basal ganglia, and is crucially involved in motor control. Striatal projection cells are medium-size spiny neurons (MSNs) and form functional GABAergic synapses with other MSNs through their axon collaterals. A subpopulation of MSNs also release substance P (SP), but its role in MSN-MSN communication is unknown. We studied this issue in rat brain slices, in the presence of antagonists for GABA, acetylcholine, dopamine, and opioid receptors; under these conditions, whole-cell paired recordings from MSNs (located <100 microm apart) revealed that, in 31/137 (23%) pairs, a burst of five spikes in a MSN caused significant facilitation (14.2 +/- 8.9%) of evoked glutamatergic responses in the other MSN. Reciprocal facilitation of glutamatergic responses was present in 4 of these pairs. These facilitatory effects were maximal when spikes preceded glutamatergic responses by 100 ms, and were completely blocked by the NK1 receptor antagonist L-732,138. Furthermore, in 31/57 (54%) MSNs, a burst of 5 antidromic stimuli delivered to MSN axons in the globus pallidus significantly potentiated glutamatergic responses evoked 250 or 500 ms later by stimulation of the corpus callosum. These effects were larger at 250 than 500 ms intervals, were completely blocked by L-732,138, and facilitated spike generation. These data demonstrate that MSNs facilitate glutamatergic inputs to neighboring MSNs through spike-released SP acting on NK1 receptors. The current view that MSNs form inhibitory networks characterized by competitive dynamics will have to be updated to incorporate the fact that groups of MSNs interact in an excitatory manner.
Figures
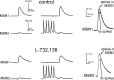

References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. - PubMed
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. - PubMed
-
- Almeida TA, Rojo J, Nieto PM, Pinto FM, Hernandez M, Martín JD, Candenas ML. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11:2045–2081. - PubMed
-
- Baranauskas G, Traversa U, Rosati AM, Nistri A. An NK1 receptor-dependent component of the slow excitation recorded intracellularly from rat motoneurons following dorsal root stimulation. Eur J Neurosci. 1995;7:2409–2417. - PubMed
-
- Bar-Gad I, Morris G, Bergman H. Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol. 2003;71:439–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
