Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs
- PMID: 19369666
- PMCID: PMC2737185
- DOI: 10.1056/NEJMoa0803531
Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs
Abstract
Background: Fetal exposure of animals to antiepileptic drugs at doses lower than those required to produce congenital malformations can produce cognitive and behavioral abnormalities, but cognitive effects of fetal exposure of humans to antiepileptic drugs are uncertain.
Methods: Between 1999 and 2004, we enrolled pregnant women with epilepsy who were taking a single antiepileptic agent (carbamazepine, lamotrigine, phenytoin, or valproate) in a prospective, observational, multicenter study in the United States and the United Kingdom. The primary analysis is a comparison of neurodevelopmental outcomes at the age of 6 years after exposure to different antiepileptic drugs in utero. This report focuses on a planned interim analysis of cognitive outcomes in 309 children at 3 years of age.
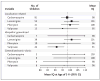
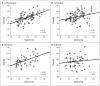
Results: At 3 years of age, children who had been exposed to valproate in utero had significantly lower IQ scores than those who had been exposed to other antiepileptic drugs. After adjustment for maternal IQ, maternal age, antiepileptic-drug dose, gestational age at birth, and maternal preconception use of folate, the mean IQ was 101 for children exposed to lamotrigine, 99 for those exposed to phenytoin, 98 for those exposed to carbamazepine, and 92 for those exposed to valproate. On average, children exposed to valproate had an IQ score 9 points lower than the score of those exposed to lamotrigine (95% confidence interval [CI], 3.1 to 14.6; P=0.009), 7 points lower than the score of those exposed to phenytoin (95% CI, 0.2 to 14.0; P=0.04), and 6 points lower than the score of those exposed to carbamazepine (95% CI, 0.6 to 12.0; P=0.04). The association between valproate use and IQ was dose dependent. Children's IQs were significantly related to maternal IQs among children exposed to carbamazepine, lamotrigine, or phenytoin but not among those exposed to valproate.
Conclusions: In utero exposure to valproate, as compared with other commonly used antiepileptic drugs, is associated with an increased risk of impaired cognitive function at 3 years of age. This finding supports a recommendation that valproate not be used as a first-choice drug in women of childbearing potential.
2009 Massachusetts Medical Society
Conflict of interest statement
Dr. Meador reports receiving research support from the McKnight Brain Institute, the MCG Foundation, GlaxoSmithKline, Eisai Medical Research, Myriad Pharmaceuticals, Marinus Pharmaceuticals, NeuroPace, SAM Technology, and UCB Pharma and serving on the professional advisory board of the Epilepsy Foundation. Dr. Baker reports serving on paid advisory boards for UCB Pharma; receiving lecture fees from Pfizer, UCB Pharma, and Janssen; receiving grant support from Sanofi-Aventis and Pfizer; and serving as an expert witness in litigation related to neurodevelopmental effects of antiepileptic drugs. Dr. Clayton-Smith reports serving as an expert witness in litigation related to neurodevelopmental effects of antiepileptic drugs. Dr. Combs-Cantrell reports receiving lecture fees from GlaxoWellcome. Dr. Kalayjian reports receiving lecture fees from GlaxoSmithKline and Ortho-McNeil and grant support from Marinus Pharmaceuticals. Dr. Kanner reports receiving lecture fees from GlaxoSmithKline, Ortho-McNeil, Pfizer, and UCB Pharma; advisory-board fees from GlaxoSmithKline, Ortho-McNeil, Valeant Pharmaceuticals, and UCB Pharma; and grant support from GlaxoSmithKline and Novartis. Dr. Liporace reports serving on the professional board of the Epilepsy Foundation of Eastern Pennsylvania. Dr. Pennell reports serving on the Keppra Pregnancy Registry Expert Panel (UCB Pharma) and receiving grant support from UCB Pharma, Marinus Pharmaceuticals, and GlaxoSmithKline. Dr. Privitera reports receiving consulting and advisory-board fees from Ortho-McNeil, UCB Pharma, Johnson & Johnson, and the National EpiFellows Foundation; lecture fees from Ortho-McNeil, Pfizer, GlaxoSmithKline, Janssen, and UCB Pharma; and grant support from UCB Pharma, Ortho-McNeil, and the American Epilepsy Society. Dr. Loring reports receiving consulting fees from UCB Pharma, NeuroPace, and Sanofi-Aventis and grant support from Myriad Pharmaceuticals, SAM Technology, and Novartis. No other potential conflict of interest relevant to this article was reported.
Figures

Comment in
-
Which drug for the pregnant woman with epilepsy?N Engl J Med. 2009 Apr 16;360(16):1667-9. doi: 10.1056/NEJMe0901550. N Engl J Med. 2009. PMID: 19369673 No abstract available.
-
Cognitive function after fetal exposure to antiepileptic drugs.N Engl J Med. 2009 Aug 6;361(6):629; author reply 629-30. doi: 10.1056/NEJMc091029. N Engl J Med. 2009. PMID: 19657130 No abstract available.
-
Fetal exposure to valproate was associated with lower IQ scores at 3 years of age than exposure to other antiepileptic drugs.Evid Based Med. 2009 Oct;14(5):153. doi: 10.1136/ebm.14.5.153. Evid Based Med. 2009. PMID: 19794028 No abstract available.
-
Cognitive function in children is differentially affected following exposure to antiepileptic drugs in utero.J Midwifery Womens Health. 2010 Jul-Aug;55(4):388-9. doi: 10.1016/j.jmwh.2010.03.013. J Midwifery Womens Health. 2010. PMID: 20630366 No abstract available.
References
-
- Pennell PB. Pregnancy in women who have epilepsy. Neurol Clin. 2004;22:799–820. - PubMed
-
- Fisher JE, Vorhees C. Developmental toxicity of antiepileptic drugs: relationship to postnatal dysfunction. Pharmacol Res. 1992;26:207–21. - PubMed
-
- Gaily E, Meador KJ. Neurodevelopmental effects. In: Engel J Jr, Pedley TA, editors. Epilepsy: a comprehensive textbook. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1225–33.
-
- Finnell RH, Nau H, Yerby MS. General principles: teratogenicity of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic drugs. 4. New York: Raven Press; 1995. pp. 209–30.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
