16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis
- PMID: 19369970
- PMCID: PMC2713796
- DOI: 10.1038/ismej.2009.37
16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis
Abstract
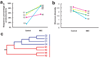
Neonatal necrotizing enterocolitis (NEC) is an inflammatory intestinal disorder affecting preterm infants. Intestinal bacteria have an important function; however no causative pathogen has been identified. The purpose of this study was to determine if there are differences in microbial patterns that may be critical to the development of this disease. Fecal samples from 20 preterm infants, 10 with NEC and 10 matched controls (including 4 twin pairs) were obtained from patients in a single site level III neonatal intensive care unit. Bacterial DNA from individual fecal samples was PCR-amplified and subjected to terminal restriction fragment length polymorphism analysis and library sequencing of the 16S rRNA gene to characterize diversity and structure of the enteric microbiota. The distribution of samples from NEC patients distinctly clustered separately from controls. Intestinal bacterial colonization in all preterm infants was notable for low diversity. Patients with NEC had even less diversity, an increase in abundance of Gammaproteobacteria, a decrease in other bacteria species, and had received a higher mean number of previous days of antibiotics. Our results suggest that NEC is associated with severe lack of microbiota diversity that may accentuate the impact of single dominant microorganisms favored by empiric and widespread use of antibiotics.
Figures




References
-
- Bell MJ, Feigin RD, Ternberg JL. Changes in the incidence of necrotizing enterocolitis associated with variation of the gastrointestinal microflora in neonates. Am J Surg. 1979;138(5):629–631. - PubMed
-
- Bell MJ, Shackelford P, Feigin RD, Ternberg JL, Brotherton T. Epidemiologic and bacteriologic evaluation of neonatal necrotizing enterocolitis. J Pediatr Surg. 1979;14(1):1–4. - PubMed
-
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147(2):192–196. - PubMed
-
- Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197(3):435–438. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- P30 DK042086/DK/NIDDK NIH HHS/United States
- HD043839/HD/NICHD NIH HHS/United States
- HD 059123/HD/NICHD NIH HHS/United States
- DK047722/DK/NIDDK NIH HHS/United States
- DK42086/DK/NIDDK NIH HHS/United States
- R37 DK047722/DK/NIDDK NIH HHS/United States
- HG4858/HG/NHGRI NIH HHS/United States
- R21 AT004044/AT/NCCIH NIH HHS/United States
- K08 HD043839/HD/NICHD NIH HHS/United States
- R01 DK047722/DK/NIDDK NIH HHS/United States
- R21 HG004858/HG/NHGRI NIH HHS/United States
- R01 HD059123/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

