Involvement of UTP in protection of cardiomyocytes from hypoxic stress
- PMID: 19370082
- PMCID: PMC3415250
- DOI: 10.1139/Y09-010
Involvement of UTP in protection of cardiomyocytes from hypoxic stress
Abstract
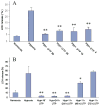
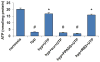
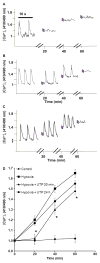
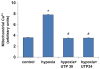
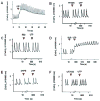
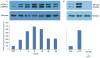
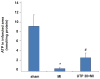
Massive amounts of nucleotides are released during ischemia in the cardiovascular system. Although the effect of the purine nucleotide ATP has been intensively studied in myocardial infarction, the cardioprotective role of the pyrimidine nucleotide UTP is still unclear, especially in the cardiovascular system. The purpose of our study was to elucidate the protective effects of UTP receptor activation and describe the downstream cascade for the cardioprotective effect. Cultured cardiomyocytes and left anterior descending (LAD)-ligated rat hearts were pretreated with UTP and exposed to hypoxia-ischemia. In vitro experiments revealed that UTP reduced cardiomyocyte death induced by hypoxia, an effect that was diminished by suramin. UTP caused several effects that could trigger a cardioprotective response: a transient increase of [Ca2+]i, an effect that was abolished by PPADS or RB2; phosphorylation of the kinases ERK and Akt, which was abolished by U0126 and LY294002, respectively; and reduced mitochondrial calcium elevation after hypoxia. In vivo experiments revealed that UTP maintained ATP levels, improved mitochondrial activity, and reduced infarct size. In conclusion, UTP administrated before ischemia reduced infarct size and improved myocardial function. Reduction of mitochondrial calcium overload can partially explain the protective effect of UTP after hypoxic-ischemic injury.
Figures




Similar articles
-
Uridine-5'-triphosphate (UTP) maintains cardiac mitochondrial function following chemical and hypoxic stress.J Mol Cell Cardiol. 2007 Nov;43(5):653-62. doi: 10.1016/j.yjmcc.2007.07.060. Epub 2007 Aug 7. J Mol Cell Cardiol. 2007. PMID: 17880998
-
Involvement of uracil nucleotides in protection of cardiomyocytes from hypoxic stress.Biochem Pharmacol. 2005 Apr 15;69(8):1215-23. doi: 10.1016/j.bcp.2005.01.018. Biochem Pharmacol. 2005. PMID: 15794942 Free PMC article.
-
Uridine-5'-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct.Biochem Pharmacol. 2006 Oct 16;72(8):949-55. doi: 10.1016/j.bcp.2006.07.019. Epub 2006 Aug 30. Biochem Pharmacol. 2006. PMID: 16939682 Free PMC article.
-
Role of carvacrol in cardioprotection against myocardial ischemia/reperfusion injury in rats through activation of MAPK/ERK and Akt/eNOS signaling pathways.Eur J Pharmacol. 2017 Feb 5;796:90-100. doi: 10.1016/j.ejphar.2016.11.053. Epub 2016 Dec 1. Eur J Pharmacol. 2017. PMID: 27916558
-
Humoral transfer and intramyocardial signal transduction of protection by remote ischemic perconditioning in pigs, rats, and mice.Am J Physiol Heart Circ Physiol. 2018 Jul 1;315(1):H159-H172. doi: 10.1152/ajpheart.00152.2018. Epub 2018 Mar 23. Am J Physiol Heart Circ Physiol. 2018. PMID: 29569956
Cited by
-
Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase.J Lipid Res. 2012 Apr;53(4):709-17. doi: 10.1194/jlr.M023424. Epub 2012 Feb 6. J Lipid Res. 2012. PMID: 22315395 Free PMC article.
-
Pyrimidinergic Receptor Activation Controls Toxoplasma gondii Infection in Macrophages.PLoS One. 2015 Jul 20;10(7):e0133502. doi: 10.1371/journal.pone.0133502. eCollection 2015. PLoS One. 2015. PMID: 26192447 Free PMC article.
-
Adenosine A(1) and A (3) receptor agonists reduce hypoxic injury through the involvement of P38 MAPK.Mol Cell Biochem. 2010 Dec;345(1-2):153-60. doi: 10.1007/s11010-010-0568-5. Epub 2010 Aug 22. Mol Cell Biochem. 2010. PMID: 20730620
-
On inotropic effects of UTP in the human heart.Heliyon. 2019 Aug 2;5(8):e02197. doi: 10.1016/j.heliyon.2019.e02197. eCollection 2019 Aug. Heliyon. 2019. PMID: 31406941 Free PMC article. Review.
-
Adenosine diphosphate reduces infarct size and improves porcine heart function after myocardial infarct.Physiol Rep. 2013 Jun;1(1):e00003. doi: 10.1002/PHY2.3. Epub 2013 May 21. Physiol Rep. 2013. PMID: 24303097 Free PMC article.
References
-
- Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. - PubMed
-
- Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Bolis L, Straub RW, editors. Cell membrane receptors for drugs and hormones: a multidisciplinary approach. Raven Press; New York: 1978. pp. 107–118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

