Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties
- PMID: 19370156
- PMCID: PMC2667250
- DOI: 10.1371/journal.pone.0005222
Chemically-induced RAT mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties
Abstract
Induction of adult rat bone marrow mesenchymal stem cells (MSC) by means of chemical compounds (beta-mercaptoethanol, dimethyl sulfoxide and butylated hydroxyanizole) has been proposed to lead to neuronal transdifferentiation, and this protocol has been broadly used by several laboratories worldwide. Only a few hours of MSC chemical induction using this protocol is sufficient for the acquisition of neuronal-like morphology and neuronal protein expression. However, given that cell death is abundant, we hypothesize that, rather than true neuronal differentiation, this particular protocol leads to cellular toxic effects. We confirm that the induced cells with neuronal-like morphology positively stained for NF-200, S100, beta-tubulin III, NSE and MAP-2 proteins. However, the morphological and molecular changes after chemical induction are also associated with an increase in the apoptosis of over 50% of the plated cells after 24 h. Moreover, increased intracellular cysteine after treatment indicates an impairment of redox circuitry during chemical induction, and in vitro electrophysiological recordings (patch-clamp) of the chemically induced MSC did not indicate neuronal properties as these cells do not exhibit Na(+) or K(+) currents and do not fire action potentials. Our findings suggest that a disruption of redox circuitry plays an important role in this specific chemical induction protocol, which might result in cytoskeletal alterations and loss of functional ion-gated channels followed by cell death. Despite the neuronal-like morphology and neural protein expression, induced rat bone marrow MSC do not have basic functional neuronal properties, although it is still plausible that other methods of induction and/or sources of MSC can achieve a successful neuronal differentiation in vitro.
Conflict of interest statement
Figures

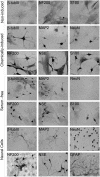


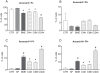



Similar articles
-
Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types.Exp Hematol. 2006 Nov;34(11):1563-72. doi: 10.1016/j.exphem.2006.06.020. Exp Hematol. 2006. PMID: 17046576
-
Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype.J Neurosci Res. 2004 Jul 15;77(2):192-204. doi: 10.1002/jnr.20147. J Neurosci Res. 2004. PMID: 15211586
-
Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact?J Neurosci Res. 2004 Jul 15;77(2):174-91. doi: 10.1002/jnr.20148. J Neurosci Res. 2004. PMID: 15211585
-
Astrocytic and neuronal fate of mesenchymal stem cells expressing nestin.Brain Res Bull. 2005 Dec 15;68(1-2):95-102. doi: 10.1016/j.brainresbull.2005.08.016. Epub 2005 Sep 21. Brain Res Bull. 2005. PMID: 16325009 Review.
-
Electrophysiological properties and synaptic function of mesenchymal stem cells during neurogenic differentiation - a mini-review.Int J Artif Organs. 2012 May;35(5):323-37. doi: 10.5301/ijao.5000085. Int J Artif Organs. 2012. PMID: 22505200 Review.
Cited by
-
Forskolin and IBMX Induce Neural Transdifferentiation of MSCs Through Downregulation of the NRSF.Sci Rep. 2019 Feb 27;9(1):2969. doi: 10.1038/s41598-019-39544-0. Sci Rep. 2019. PMID: 30814572 Free PMC article.
-
Expression of the chitinase family glycoprotein YKL-40 in undifferentiated, differentiated and trans-differentiated mesenchymal stem cells.PLoS One. 2013 May 9;8(5):e62491. doi: 10.1371/journal.pone.0062491. Print 2013. PLoS One. 2013. PMID: 23671604 Free PMC article.
-
Biology and applications of mesenchymal stem cells.Sci Prog. 2010;93(Pt 2):113-27. doi: 10.3184/003685010x12708175591515. Sci Prog. 2010. PMID: 20681317 Free PMC article. Review.
-
Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells.J R Soc Interface. 2011 Jul 6;8(60):998-1010. doi: 10.1098/rsif.2010.0613. Epub 2011 Jan 19. J R Soc Interface. 2011. PMID: 21247946 Free PMC article.
-
Adipose tissue-derived stromal cells (ADSC) express oligodendrocyte and myelin markers, but they do not function as oligodendrocytes.Histochem Cell Biol. 2017 Nov;148(5):503-515. doi: 10.1007/s00418-017-1588-y. Epub 2017 Jun 15. Histochem Cell Biol. 2017. PMID: 28620864
References
-
- Abouelfetouh A, Kondoh T, Ehara K, Kohmura E. Morphological differentiation of bone marrow stromal cells into neuron-like cells after co-culture with hippocampal slice. Brain Res. 2004;1029:114–119. - PubMed
-
- Bonilla S, Silva A, Valdes L, Geijo E, Garcia-Verdugo JM, et al. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–95. - PubMed
-
- Bossolasco P, Cova L, Calzarossa C, Rimoldi SG, Borsotti C, et al. Neuro-glial differentiation of human bone marrow stem cells in vitro. Exp Neurol. 2005;193:312–325. - PubMed
-
- Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. - PubMed
-
- Lei Z, Yongda L, Jun M, Yingyu S, Shaoju Z, et al. Culture and neural differentiation of rat bone marrow mesenchymal stem cells in vitro. Cell Biol Int. 2007;31:916–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

