Homeopathic medicines for adverse effects of cancer treatments
- PMID: 19370613
- PMCID: PMC10422695
- DOI: 10.1002/14651858.CD004845.pub2
Homeopathic medicines for adverse effects of cancer treatments
Abstract
Background: Homeopathic medicines are used by patients with cancer, often alongside conventional treatment. Cancer treatments can cause considerable morbidity and one of the reasons patients use homeopathic medicines is to help with adverse effects.
Objectives: Evaluate effectiveness and safety of homeopathic medicines used to prevent or treat adverse effects of cancer treatments.
Search strategy: The following were searched up to November 2008: Cochrane PaPaS Trials Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; CINAHL; BNI; CancerLIT; AMED; CISCOM; Hom-Inform; SIGLE; National Research Register; Zetoc; www.controlled-trials.com; http://clinicaltrials.gov; Liga Medicorum Homeopathica Internationalis (LMHI, Liga) conference proceedings; reference lists of relevant studies were checked; and homeopathic manufacturers, leading researchers and practitioners were contacted.
Selection criteria: Randomised controlled trials (RCTs) of homeopathic medicines in participants with a clinical or histological diagnosis of cancer where the intervention was aimed at preventing or treating symptoms associated with cancer treatments. All age groups, and all stages of disease were included.
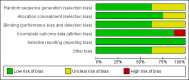
Data collection and analysis: Two review authors independently assessed studies for inclusion and two review authors extracted data. Three review authors independently assessed trial quality using the Delphi List and the Cochrane Collaboration's tool for assessing risk of bias. Disagreements were resolved by consensus. Where available, data were extracted for analysis.
Main results: Eight controlled trials (seven placebo controlled and one trial against an active treatment) with a total of 664 participants met the inclusion criteria. Three studied adverse effects of radiotherapy, three studied adverse effects of chemotherapy and two studied menopausal symptoms associated with breast cancer treatment.Two studies with low risk of bias demonstrated benefit: one with 254 participants demonstrated superiority of topical calendula over trolamine (a topical agent not containing corticosteroids) for prevention of radiotherapy-induced dermatitis, and another with 32 participants demonstrated superiority of Traumeel S (a proprietary complex homeopathic medicine) over placebo as a mouthwash for chemotherapy-induced stomatitis. Two other studies reported positive results, although the risk of bias was unclear, and four further studies reported negative results.No serious adverse effects or interactions were reported attributable to the homeopathic medicines used.
Authors' conclusions: This review found preliminary data in support of the efficacy of topical calendula for prophylaxis of acute dermatitis during radiotherapy and Traumeel S mouthwash in the treatment of chemotherapy-induced stomatitis. These trials need replicating. There is no convincing evidence for the efficacy of homeopathic medicines for other adverse effects of cancer treatments. Further research is required.
Conflict of interest statement
Peter Fisher has received fees from homeopathic manufactures for lectures and seminars.
Sosie Kassab is Director of Complementary Cancer Services at the Royal London Homoeopathic Hospital and uses homeopathic medicines for patients with cancer alongside their conventional care.
Robbert van Haselen was Deputy Director of Research at the Royal London Homoeopathic Hospital when an application for funding for this Cochrane Review was made from ViFAB. He had a major input into the development of the protocol which was published in 2004. He left the hospital in 2005 and took up his post as Director of Research for Heel in Germany in 2006 (the company that makes Traumeel S, one of the interventions included in this review). Prior to his leaving, we had run some of the searches and identified some potential studies but had not gone through the process of formally selecting studies for inclusion into the review. He had no input into the selection of included studies, data extraction, quality assessment or interpretation of the analysis. On finally approving the publication, he did not make any recommendations for change to the implications for clinical practice, research or to the conclusions, but commented on it critically for intellectual content.
Figures

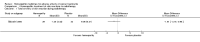
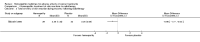
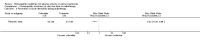
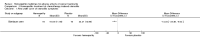
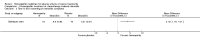

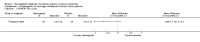
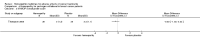

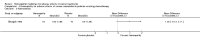
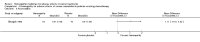
Comment in
-
Extracts from the Cochrane Library: homeopathic medicines for adverse effects of cancer treatments.Otolaryngol Head Neck Surg. 2009 Aug;141(2):162-5. doi: 10.1016/j.otohns.2009.06.081. Otolaryngol Head Neck Surg. 2009. PMID: 19643245 No abstract available.
References
References to studies included in this review
Balzarini 2000 {published data only}
-
- Balzarini A, Felisi E, Martini A, Conno F. Efficacy of homeopathic treatment of skin reactions during radiotherapy for breast cancer: a randomised, double‐blind clinical trial. British Homeopathic Journal 2000;89(1):8‐12. - PubMed
Bourgois 1984 {published data only}
-
- Bourgois JC. Protection of the venous capital at the perfusees with a long course in breast cancer. Double blind Clinical trial: Arnica with a placebo control [Protection du capital veineux chez les perfusees au long cours dans cancer du sein. Essai clinique en double aveugle: Arnica contro placebo]. Thesis 1984:89.
Daub 2005 {published data only}
-
- Daub EA, Gerhard I, Bastert G. Homeopathic antiemesis for chemotherapy, a prospective randomised trial [Homoopathische Antiemetika bei Chemotherapie, eine prospektiv, randomisierte Studie]. Geburtshilfe und Frauenheilkunde 2005;60:S157.
Jacobs 2005 {published data only}
-
- Jacobs J, Herman P, Heron K, Olsen S, Vaughters L. Homeopathy for menopausal symptoms in breast cancer survivors: a preliminary randomized controlled trial. Journal of Alternative and Complementary Medicine 2005;11(1):21‐7. - PubMed
Kulkarni 1988 {published data only}
-
- Kulkarni A, Nagarkar BM, Burde GS. Radiation protection by use of homoeopathic medicines. Hahnemann Homoeopath Sand 1988;12:20‐3.
Oberbaum 2001 {published data only}
-
- Oberbaum M, Yaniv I, Ben Gal Y, Stein J, Ben Zvi N, Freedman LS, et al. A randomized, controlled clinical trial of the homeopathic medication TRAUMEEL S in the treatment of chemotherapy‐induced stomatitis in children undergoing stem cell transplantation. Cancer 2001;92(3):684‐90. - PubMed
Pommier 2004 {published data only}
-
- Pommier P, Gomez F, Sunyach MP, D'Hombres A, Carrie C, Montbarbon X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. Journal of Clinical Oncology 2004;22(8):1447‐53. - PubMed
Thompson 2005 {published data only}
-
- Thompson EA, Montgomery A, Douglas D, Reilly D. A pilot, randomized, double‐blinded, placebo‐controlled trial of individualized homeopathy for symptoms of estrogen withdrawal in breast‐cancer survivors. Journal of Alternative and Complementary Medicine 2005;11(1):13‐20. - PubMed
References to studies excluded from this review
Felisi 1994 {published data only}
-
- Felisi E, Saruggia M, Russo G, Balzarini A. Early cutaneous lesions raised during radiotherapy whilst treating breast cancer: Effectiveness of a homeopathic therapy [Lésions cutanées précoces relevées au cours de radiothérapie misant au traitment du cancer du sein: L'efficacité d'une thérapie homéopathique]. OMHI Congress. 1994.
Srihari 1995 {published data only}
-
- Srihari U. The Ipecac trial. Proceedings of the 49th Liga Medicorum Homoeopathica Internationalis Congress. New Delhi, 1995; Vol. 1:307‐12.
References to ongoing studies
Genre 2003 {published data only}
-
- Genre D, Tarpin C, Braud AC, Camerlo J, Protiere C, Eisinger F, et al. Randomized, double‐blind study comparing homeopathy (cocculine) to placebo in prevention of nausea/vomiting among patients receiving adjuvant chemotherapy for breast cancer. Breast Cancer Research and Treatment 2003;82:637.
Krempl 2005 {published data only}
-
- Krempl G. A trial of homeopathic medication Traumeel S for the treatment of radiation‐induced mucositis. http://clinicaltrials.gov/show/NCT00584597 Accessed February 2008.
Ray‐Coquard 2005 {published data only}
-
- Placebo‐controlled evaluation of Cocculine® efficacy in the management of nausea after chemotherapy in breast cancer. http://clinicaltrials.gov/show/NCT00409071 Accessed February 2008.
Sadhev 2004 {published data only}
-
- Traumeel® S in preventing and treating mucositis in young patients undergoing stem cell transplantation. http://clinicaltrials.gov/ct2/show/NCT00080873 Accessed February 2008.
Additional references
Bell 2003
-
- Bell I. Evidence‐based homeopathy: empirical questions and methodological considerations for homeopathic clinical research. American Journal of Homeopathic Medicine 2003;96(1):17‐31.
Dantas 2000
-
- Dantas F, Rampes H. Do homeopathic medicines provoke adverse effects? A systematic review'. British Homeopathic Journal 2000;89(Suppl 1):35‐8. - PubMed
Ernst 1998
-
- Ernst E, Cassileth BR. The prevalence of complementary and alternative medicine in cancer: a systematic review. Cancer 1998;83:777‐82. - PubMed
Fisher 1998
-
- Fisher P. The information medicine hypothesis. In: Shulte J, Endler PC editor(s). Fundamental research in high dilutions. Kluwer Dordrecht, 1998:xi‐xiv.
Higgins 2008
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008.
Linde 1997
-
- Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges L, Jonas W. Are the clinical effects of homeopathy placebo effects? A meta‐analysis of placebo controlled trials. Lancet 1997;350:834‐43. - PubMed
Milazzo 2006
-
- Milazzo S, Russell N, Ernst E. Efficacy of homeopathic therapy in cancer treatment. European Journal of Cancer 2006;42(3):282‐9. - PubMed
Molassiotis 2005
-
- Molassiotis A, Fernadez‐Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Annals of Oncology 2005;16(4):655‐63. - PubMed
Verhagen 1998
-
- Verhagen AP, Vet HCW, Bie RA, Kessels AGH, Boers M, Bouter LM, Knipschild PG. The Delphi List: a criteria list for quality assessment developed by Delphi consensus of randomised clinical trials for conducting systematic reviews. Journal of Clinical Epidemiology 1998;51(12):1235‐41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

