Escitalopram versus other antidepressive agents for depression
- PMID: 19370639
- PMCID: PMC4164382
- DOI: 10.1002/14651858.CD006532.pub2
Escitalopram versus other antidepressive agents for depression
Abstract
Background: Although pharmacological and psychological interventions are both effective for major depression, antidepressant drugs remain the mainstay of treatment in primary and secondary care settings. During the last 20 years, antidepressant prescribing has risen dramatically in western countries, mainly because of the increasing consumption of selective serotonin reuptake inhibitors (SSRIs) and newer antidepressants, which have progressively become the most commonly prescribed antidepressants. Escitalopram is the pure S-enantiomer of the racemic citalopram.
Objectives: To assess the evidence for the efficacy, acceptability and tolerability of escitalopram in comparison with tricyclics, other SSRIs, heterocyclics and newer agents in the acute-phase treatment of major depression.
Search strategy: Electronic databases were searched up to July 2008. Trial databases of drug-approving agencies were hand-searched for published, unpublished and ongoing controlled trials.
Selection criteria: All randomised controlled trials comparing escitalopram against any other antidepressant (including non-conventional agents such as hypericum) for patients with major depressive disorder (regardless of the diagnostic criteria used).
Data collection and analysis: Data were entered by two review authors (double data entry). Responders and remitters to treatment were calculated on an intention-to-treat basis. For dichotomous data, odds ratios (ORs) were calculated with 95% confidence intervals (CI). Continuous data were analysed using standardised mean differences (with 95% CI) using the random effects model.
Main results: Fourteen trials compared escitalopram with another SSRI and eight compared escitalopram with a newer antidepressive agent (venlafaxine, bupropion and duloxetine). Escitalopram was shown to be significantly more effective than citalopram in achieving acute response (OR 0.67, 95% CI 0.50 to 0.87). Escitalopram was also more effective than citalopram in terms of remission (OR 0.53, 95% CI 0.30 to 0.93). Significantly fewer patients allocated to escitalopram withdrew from trials compared with patients allocated to duloxetine, for discontinuation due to any cause (OR 0.62, 95% CI 0.38 to 0.99).
Authors' conclusions: Some statistically significant differences favouring escitalopram over other antidepressive agents for the acute phase treatment of major depression were found, in terms of efficacy (citalopram and fluoxetine) and acceptability (duloxetine). There is insufficient evidence to detect a difference between escitalopram and other antidepressants in early response to treatment (after two weeks of treatment). Cost-effectiveness information is also needed in the field of antidepressant trials. Furthermore, as with most standard systematic reviews, the findings rely on evidence from direct comparisons. The potential for overestimation of treatment effect due to sponsorship bias should also be borne in mind.
Figures
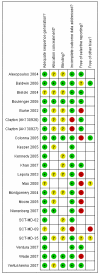





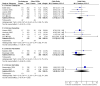






References
References to studies included in this review
-
- Alexopoulos G, Gordon J, Zhang D. A placebo-controlled trial of escitalopram and sertraline in the treatment of major depressive disorder; Poster presented at the Annual Meeting of the American College of Neuropsychopharmacology; 2004.Dec,
-
- Baldwin S, Cooper JA, Huusom AKT, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. International Clinical Psychopharmacology. 2006;21(3):159–169. - PubMed
-
- Bielski RJ, Ventura D, Chung-Chi Chang. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. Journal of Clinical Psychiatry. 2004;65(9):1190–1196. - PubMed
-
- Boulenger J-P, Huusom AKT, Florea I, Baekdal T, Sarchiapone M. A comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severly depressed patients. Current Medical Research and Opinion. 2006;22(7):1331–1341. - PubMed
-
- Burke WJ, Gergel I, Bose A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. Journal of Clinical Psychiatry. 2002;63(4):331–336. - PubMed
Additional references
-
- Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA. 2003;290:921–8. - PubMed
-
- Anderson IM, Nutt DJ, Deakin JF. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 1993 British Association for Psychopharmacology guidelines. Journal of Psychopharmacology. 2000;14:3–20. - PubMed
-
- American Psychiatric Association . Diagnostical and Statistical Manual of Mental Disorders (DSM-III) 3rd Edition APA; Washington, DC: 1980.
-
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) 3rd Edition American Psychiatric Association; Washington, DC: 1987.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

