Influence of food intake on the bioavailability and efficacy of oral calcitonin
- PMID: 19371314
- PMCID: PMC2679104
- DOI: 10.1111/j.1365-2125.2009.03371.x
Influence of food intake on the bioavailability and efficacy of oral calcitonin
Abstract
Aims: To investigate the influence of food intake on the bioavailability and pharmacodynamic effects of salmon calcitonin (sCT).
Methods: A single-blind, randomized, partly placebo-controlled study was conducted in 36 healthy postmenopausal female volunteers aged 62-74 years. The influence of food intake on oral dosing with 0.8 mg of sCT at 22.00 h was evaluated for a (i) predose meal at 18.00 h, (ii) predose meal at 20.00 h, (iii) predose meal at 21.00 h, (iv) postdose meal at 22.10 h, (v) no meal, and (vi) meal at 20.00 h and placebo at 22.00 h. Study biomarkers were plasma sCT levels and changes in the bone resorption marker CTX-I (C-terminal telopeptide of collagen type I).
Results: The predose meal at 18.00 and 21.00 h significantly decreased relative oral bioavailability of sCT to 26% [95% confidence interval (CI) 0.09, 0.73 and 0.09, 0.75, P= 0.009 and P= 0.01]. The meal consumed 10 min after dosing decreased the oral bioavailability of sCT to 59% (95% CI 0.21, 1.68), although nonsignificant (P= 0.48). This decreased bioavailability led to lower relative suppression of serum CTX-I, with an AUC of the 4-h efficacy response of -91%-x-hours for those receiving a meal at 18.00 h, compared with -238%-x-hours for fasting subjects. The Dunnett-adjusted difference between these two treatment sequences was 147%-x-hours (95% CI 68, 225) (P= 0.0003). The AUC was comparable among fasting subjects and those consuming a meal 10 min after dosing.
Conclusions: Postprandial dosing may limit the bioavailability of orally administered sCT. Maximal benefit can be achieved by dosing at least 10 min prior to meal time.
Figures
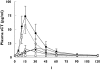
 ); IV: Meal at 22:10 (--○--); V: No meal (—•—); VI: Placebo (--□--)
); IV: Meal at 22:10 (--○--); V: No meal (—•—); VI: Placebo (--□--)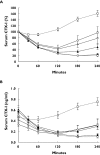
 ); IV: Meal at 22:10 (--○--); V: No meal (—•—); VI: Placebo (--□--)
); IV: Meal at 22:10 (--○--); V: No meal (—•—); VI: Placebo (--□--)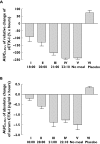
Similar articles
-
Optimizing bioavailability of oral administration of small peptides through pharmacokinetic and pharmacodynamic parameters: the effect of water and timing of meal intake on oral delivery of Salmon Calcitonin.BMC Clin Pharmacol. 2008 Sep 9;8:5. doi: 10.1186/1472-6904-8-5. BMC Clin Pharmacol. 2008. PMID: 18782439 Free PMC article. Clinical Trial.
-
Investigations of inter- and intraindividual relationships between exposure to oral salmon calcitonin and a surrogate marker of pharmacodynamic efficacy.Eur J Clin Pharmacol. 2010 Jan;66(1):29-37. doi: 10.1007/s00228-009-0735-3. Epub 2009 Oct 8. Eur J Clin Pharmacol. 2010. PMID: 19813008 Clinical Trial.
-
A pharmacokinetic and pharmacodynamic comparison of synthetic and recombinant oral salmon calcitonin.J Clin Pharmacol. 2009 Feb;49(2):229-34. doi: 10.1177/0091270008329552. J Clin Pharmacol. 2009. PMID: 19179298 Clinical Trial.
-
Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in the treatment of osteoporosis.Expert Opin Drug Metab Toxicol. 2016 Jun;12(6):681-9. doi: 10.1080/17425255.2016.1175436. Epub 2016 Apr 18. Expert Opin Drug Metab Toxicol. 2016. PMID: 27070719 Review.
-
Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials.J Clin Pharmacol. 2011 Apr;51(4):460-71. doi: 10.1177/0091270010372625. Epub 2010 Jul 21. J Clin Pharmacol. 2011. PMID: 20660294 Review.
Cited by
-
Will posttranslational modifications of brain proteins provide novel serological markers for dementias?Int J Alzheimers Dis. 2012;2012:209409. doi: 10.1155/2012/209409. Epub 2012 Jun 21. Int J Alzheimers Dis. 2012. PMID: 22779024 Free PMC article.
-
The future of blood-based biomarkers for Alzheimer's disease.Alzheimers Dement. 2014 Jan;10(1):115-31. doi: 10.1016/j.jalz.2013.01.013. Epub 2013 Jul 11. Alzheimers Dement. 2014. PMID: 23850333 Free PMC article. Review.
-
Effect of Various Dosing Conditions on the Pharmacokinetics of Oral Semaglutide, a Human Glucagon-Like Peptide-1 Analogue in a Tablet Formulation.Diabetes Ther. 2021 Jul;12(7):1915-1927. doi: 10.1007/s13300-021-01078-y. Epub 2021 Jun 2. Diabetes Ther. 2021. PMID: 34080123 Free PMC article.
-
Biochemical markers of ongoing joint damage in rheumatoid arthritis--current and future applications, limitations and opportunities.Arthritis Res Ther. 2011 Apr 28;13(2):215. doi: 10.1186/ar3280. Arthritis Res Ther. 2011. PMID: 21539724 Free PMC article. Review.
-
The Effect of Food Intake on the Pharmacokinetics of Oral Basal Insulin: A Randomised Crossover Trial in Healthy Male Subjects.Clin Pharmacokinet. 2019 Nov;58(11):1497-1504. doi: 10.1007/s40262-019-00772-2. Clin Pharmacokinet. 2019. PMID: 31093929 Free PMC article. Clinical Trial.
References
-
- Copp DH, Cameron EC, Cheney BA, Davidson AG, Henze KG. Evidence for calcitonin – a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 1962;70:638–49. - PubMed
-
- Kumar MA, Foster GV, MacIntyre I. Further evidence for calcitonin. A rapid-acting hormone which lowers plasma-calcium. Lancet. 1963;35:480–2. - PubMed
-
- Chambers TJ, Moore A. The sensitivity of isolated osteoclasts to morphological transformation by calcitonin. J Clin Endocrinol Metab. 1983;57:819–24. - PubMed
-
- Suzuki H, Nakamura I, Takahashi N, Ikuhara T, Matsuzaki K, Isogai Y, Hori M, Suda T. Calcitonin-induced changes in the cytoskeleton are mediated by a signal pathway associated with protein kinase A in osteoclasts. Endocrinology. 1996;137:4685–90. - PubMed
-
- Shyu JF, Shih C, Tseng CY, Lin CH, Sun DT, Liu HT, Tsung HC, Chen TH, Lu RB. Calcitonin induces podosome disassembly and detachment of osteoclasts by modulating Pyk2 and Src activities. Bone. 2007;40:1329–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

