Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects
- PMID: 19371315
- PMCID: PMC2679105
- DOI: 10.1111/j.1365-2125.2009.03370.x
Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects
Abstract
Aims: To characterize the impact of potent CYP3A4 inhibition and induction on lapatinib pharmacokinetics.
Methods: Two studies were conducted in healthy subjects. One study examined the effect of ketoconazole 200 mg b.i.d. for 7 days on a single 100-mg dose of lapatinib in 22 healthy subjects. The other study examined the effect of carbamazepine titrated up to 200 mg b.i.d. over 20 days on a single 250-mg dose of lapatinib in 24 healthy subjects.
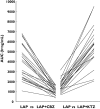
Results: Ketoconazole altered lapatinib AUC, C(max) and half-life, with geometric mean [95% confidence interval (CI)] increases of 3.57-fold (3.07, 4.15), 2.14-fold (1.74, 2.64) and 1.66-fold (1.50, 1.84), respectively, but had no effect on absorption rate. Carbamazepine altered lapatinib AUC, C(max) and absorption rate, with geometric mean (95% CI) decreases of 72% (68, 77), 59% (49, 66) and 28% (4, 46), respectively, but had no effect on half-life.
Conclusions: Systemic exposure to lapatinib was significantly altered by potent inhibition and induction of CYP3A4. Dose adjustments may be required when lapatinib is administered with orally administered drugs that potently alter the activity of this enzyme.
Figures
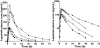
Similar articles
-
Evaluation of Cytochrome P450 (CYP) 3A4-Based Interactions of Levomilnacipran with Ketoconazole, Carbamazepine or Alprazolam in Healthy Subjects.Clin Drug Investig. 2015 Oct;35(10):601-12. doi: 10.1007/s40261-015-0318-2. Clin Drug Investig. 2015. PMID: 26315684 Clinical Trial.
-
Pharmacokinetics of oral neratinib during co-administration of ketoconazole in healthy subjects.Br J Clin Pharmacol. 2011 Apr;71(4):522-7. doi: 10.1111/j.1365-2125.2010.03845.x. Br J Clin Pharmacol. 2011. PMID: 21395644 Free PMC article. Clinical Trial.
-
The effect on sotrastaurin pharmacokinetics of strong CYP3A inhibition by ketoconazole.Br J Clin Pharmacol. 2009 Sep;68(3):381-5. doi: 10.1111/j.1365-2125.2009.03457.x. Br J Clin Pharmacol. 2009. PMID: 19740395 Free PMC article.
-
Influence of CYP3A4 induction/inhibition on the pharmacokinetics of vilazodone in healthy subjects.Clin Ther. 2014 Nov 1;36(11):1638-49. doi: 10.1016/j.clinthera.2014.08.003. Epub 2014 Sep 16. Clin Ther. 2014. PMID: 25236915 Clinical Trial.
-
Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases.Clin Ther. 2008 Aug;30(8):1426-47. doi: 10.1016/j.clinthera.2008.08.008. Clin Ther. 2008. PMID: 18803986 Review.
Cited by
-
Cytochrome P450 3A4 and CYP3A5-Catalyzed Bioactivation of Lapatinib.Drug Metab Dispos. 2016 Oct;44(10):1584-97. doi: 10.1124/dmd.116.070839. Epub 2016 Jul 22. Drug Metab Dispos. 2016. PMID: 27450182 Free PMC article.
-
Pivotal Considerations for Optimal Deployment of Healthy Volunteers in Oncology Drug Development.Clin Transl Sci. 2020 Jan;13(1):31-40. doi: 10.1111/cts.12703. Epub 2019 Oct 31. Clin Transl Sci. 2020. PMID: 31674150 Free PMC article. Review.
-
Physiologically based pharmacokinetic model of lapatinib developed in mice and scaled to humans.J Pharmacokinet Pharmacodyn. 2013 Apr;40(2):157-76. doi: 10.1007/s10928-012-9295-8. Epub 2013 Jan 12. J Pharmacokinet Pharmacodyn. 2013. PMID: 23315145 Free PMC article.
-
Structural Perspectives of the CYP3A Family and Their Small Molecule Modulators in Drug Metabolism.Liver Res. 2019 Dec;3(3-4):132-142. doi: 10.1016/j.livres.2019.08.001. Epub 2019 Aug 29. Liver Res. 2019. PMID: 32789028 Free PMC article.
-
Mechanism-based inactivation of CYP450 enzymes: a case study of lapatinib.Drug Metab Rev. 2015 Feb;47(1):21-8. doi: 10.3109/03602532.2014.1003648. Epub 2015 Feb 2. Drug Metab Rev. 2015. PMID: 25639891 Free PMC article. Review.
References
-
- Bence AK, Anderson EB, Halepota MA, Doukas MA, DeSimone PA, Davis GA, Smith DA, Koch KM, Stead AG, Mangum S, Bowen CJ, Spector NL, Hsieh S, Adams VR. Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR-erbb2 inhibitor, in healthy subjects. Invest New Drugs. 2005;23:39–49. - PubMed
-
- Reddy N, Cohen R, Whitehead B, Koch KM, Stead A, Beelen AP, Lewis LD. A phase I, open-label, three-period, randomized crossover study to evaluate the effect of food on the pharmacokinetics of lapatinib in cancer patients. Clin Pharmacol Ther. 2007;81:S16–7.
-
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, St John-Williams L, Koch KM, Serabjit-Singh CJ. The role of efflux and uptake transporters in N-{3-Chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl) ethyl]amino}methyl)-2-furyl]-4-quinazolinamine) (GW572016, Lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:1–7. - PubMed
-
- Hsieh S, Tobien T, Koch K, Dunn J. Increasing throughput of parallel on-line extraction liquid chromatography/ electrospray ionization tandem mass spectrometry system for GLP quantitative bioanalysis in drug development. Rapid Commun Mass Spectrom. 2004;18:285–92. - PubMed
-
- Gibbs MA, Thummel KE, Shen DD, Kunze KL. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos. 1999;27:180–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

