Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects
- PMID: 19371315
- PMCID: PMC2679105
- DOI: 10.1111/j.1365-2125.2009.03370.x
Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects
Abstract
Aims: To characterize the impact of potent CYP3A4 inhibition and induction on lapatinib pharmacokinetics.
Methods: Two studies were conducted in healthy subjects. One study examined the effect of ketoconazole 200 mg b.i.d. for 7 days on a single 100-mg dose of lapatinib in 22 healthy subjects. The other study examined the effect of carbamazepine titrated up to 200 mg b.i.d. over 20 days on a single 250-mg dose of lapatinib in 24 healthy subjects.
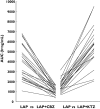
Results: Ketoconazole altered lapatinib AUC, C(max) and half-life, with geometric mean [95% confidence interval (CI)] increases of 3.57-fold (3.07, 4.15), 2.14-fold (1.74, 2.64) and 1.66-fold (1.50, 1.84), respectively, but had no effect on absorption rate. Carbamazepine altered lapatinib AUC, C(max) and absorption rate, with geometric mean (95% CI) decreases of 72% (68, 77), 59% (49, 66) and 28% (4, 46), respectively, but had no effect on half-life.
Conclusions: Systemic exposure to lapatinib was significantly altered by potent inhibition and induction of CYP3A4. Dose adjustments may be required when lapatinib is administered with orally administered drugs that potently alter the activity of this enzyme.
Figures
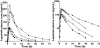
References
-
- Bence AK, Anderson EB, Halepota MA, Doukas MA, DeSimone PA, Davis GA, Smith DA, Koch KM, Stead AG, Mangum S, Bowen CJ, Spector NL, Hsieh S, Adams VR. Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR-erbb2 inhibitor, in healthy subjects. Invest New Drugs. 2005;23:39–49. - PubMed
-
- Reddy N, Cohen R, Whitehead B, Koch KM, Stead A, Beelen AP, Lewis LD. A phase I, open-label, three-period, randomized crossover study to evaluate the effect of food on the pharmacokinetics of lapatinib in cancer patients. Clin Pharmacol Ther. 2007;81:S16–7.
-
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O’Mara MJ, Olson KL, St John-Williams L, Koch KM, Serabjit-Singh CJ. The role of efflux and uptake transporters in N-{3-Chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl) ethyl]amino}methyl)-2-furyl]-4-quinazolinamine) (GW572016, Lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:1–7. - PubMed
-
- Hsieh S, Tobien T, Koch K, Dunn J. Increasing throughput of parallel on-line extraction liquid chromatography/ electrospray ionization tandem mass spectrometry system for GLP quantitative bioanalysis in drug development. Rapid Commun Mass Spectrom. 2004;18:285–92. - PubMed
-
- Gibbs MA, Thummel KE, Shen DD, Kunze KL. Inhibition of cytochrome P-450 3A (CYP3A) in human intestinal and liver microsomes: comparison of Ki values and impact of CYP3A5 expression. Drug Metab Dispos. 1999;27:180–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

