CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients
- PMID: 19371316
- PMCID: PMC2679106
- DOI: 10.1111/j.1365-2125.2009.03368.x
CYP2B6 (c.516G-->T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients
Abstract
Aims: Interindividual variability in efavirenz pharmacokinetics is not entirely explained by the well-recognized CYP2B6 516G-->T single nucleotide polymorphism. The aim of this study was to determine whether polymorphisms in the CYP2A6 gene can be used to enhance the predictability of efavirenz concentrations in human immunodeficiency virus (HIV)-infected native African patients.
Methods: Mid-dose efavirenz plasma concentrations were determined at 4 and 8 weeks following initiation of antiretroviral therapy in 65 HIV-infected Ghanaian patients. Selected CYP2B6 and CYP2A6 genotypes were determined by commercial 5'-nuclease assays. Relationships between averaged 4- and 8-week mid-dose efavirenz concentrations, demographic variables and genotypes were evaluated by univariate and multivariate statistical approaches including gene-gene interactions.
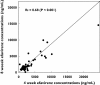
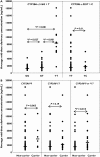
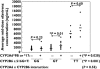
Results: CYP2B6 c.516G-->T, CYP2B6 c.983T-->C, CYP2A6*9B and CYP2A6*17 allele frequencies were 45, 4, 5 and 12%, respectively. Rifampicin therapy, gender, age and body mass index had no significant influence on efavirenz mid-dose concentrations. Median efavirenz concentrations were more than five times higher (P < 0.001) in patients with CYP2B6 c.516TT genotype compared with GG and GT genotypes. Although none of the CYP2A6 genotypes was associated with altered efavirenz concentrations individually, CYP2A6*9B and/or CYP2A6*17 carriers showed a 1.8 times higher median efavirenz concentration (P= 0.017) compared with noncarriers. Multiple linear regression analysis indicated that the CYP2B6 c.516G-->T polymorphism and CYP2A6 slow-metabolizing variants accounted for as much as 36 and 12% of the total variance in efavirenz concentrations, respectively.
Conclusions: Our findings support previous work showing efavirenz oxidation by CYP2A6, and suggest that both CYP2A6 and CYP2B6 genotyping may be useful for predicting efavirenz plasma concentrations.
Figures
Similar articles
-
CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients.AIDS. 2009 Oct 23;23(16):2101-6. doi: 10.1097/QAD.0b013e3283319908. AIDS. 2009. PMID: 19779319 Free PMC article.
-
Pharmacogenetic associations with plasma efavirenz concentrations and clinical correlates in a retrospective cohort of Ghanaian HIV-infected patients.J Antimicrob Chemother. 2014 Feb;69(2):491-9. doi: 10.1093/jac/dkt372. Epub 2013 Sep 29. J Antimicrob Chemother. 2014. PMID: 24080498
-
Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients.J Antimicrob Chemother. 2008 Apr;61(4):914-8. doi: 10.1093/jac/dkn029. Epub 2008 Feb 14. J Antimicrob Chemother. 2008. PMID: 18281305 Free PMC article.
-
Efavirenz Metabolism: Influence of Polymorphic CYP2B6 Variants and Stereochemistry.Drug Metab Dispos. 2019 Oct;47(10):1195-1205. doi: 10.1124/dmd.119.086348. Epub 2019 Jul 19. Drug Metab Dispos. 2019. PMID: 31324697 Free PMC article. Review.
-
Efavirenz in the therapy of HIV infection.Expert Opin Drug Metab Toxicol. 2010 Jan;6(1):95-103. doi: 10.1517/17425250903483207. Expert Opin Drug Metab Toxicol. 2010. PMID: 20001610 Free PMC article. Review.
Cited by
-
Efavirenz: History, Development and Future.Biomolecules. 2022 Dec 31;13(1):88. doi: 10.3390/biom13010088. Biomolecules. 2022. PMID: 36671473 Free PMC article. Review.
-
Population pharmacogenetic-based pharmacokinetic modeling of efavirenz, 7-hydroxy- and 8-hydroxyefavirenz.J Clin Pharmacol. 2014 Jan;54(1):87-96. doi: 10.1002/jcph.208. Epub 2013 Nov 19. J Clin Pharmacol. 2014. PMID: 24142869 Free PMC article. Clinical Trial.
-
Physiologically based pharmacokinetic model of lapatinib developed in mice and scaled to humans.J Pharmacokinet Pharmacodyn. 2013 Apr;40(2):157-76. doi: 10.1007/s10928-012-9295-8. Epub 2013 Jan 12. J Pharmacokinet Pharmacodyn. 2013. PMID: 23315145 Free PMC article.
-
Pharmacokinetics of Efavirenz 600 mg Once Daily During Pregnancy and Post Partum in Ghanaian Women Living With HIV.Clin Ther. 2020 Sep;42(9):1818-1825. doi: 10.1016/j.clinthera.2020.07.008. Epub 2020 Aug 15. Clin Ther. 2020. PMID: 32811669 Free PMC article.
-
Pharmacogenetics of efavirenz discontinuation for reported central nervous system symptoms appears to differ by race.Pharmacogenet Genomics. 2016 Oct;26(10):473-80. doi: 10.1097/FPC.0000000000000238. Pharmacogenet Genomics. 2016. PMID: 27509478 Free PMC article.
References
-
- US Department of Health and Human Services (DHHS) Panel on Antiretroviral Guideline for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [last accessed 20 October 2008]. 29 January 2008. Available at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
-
- Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, Cahn P, Fischl MA, Gatell JM, Hirsch MS, Jacobsen DM, Montaner JS, Richman DD, Yeni PG, Volberding PA. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–70. - PubMed
-
- Arribas JR, Pozniak AL, Gallant JE, Dejesus E, Gazzard B, Campo RE, Chen SS, McColl D, Holmes CB, Enejosa J, Toole JJ, Cheng AK. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74–8. - PubMed
-
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. - PubMed
-
- Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

