Drug targets for traumatic brain injury from poly(ADP-ribose)polymerase pathway modulation
- PMID: 19371326
- PMCID: PMC2721255
- DOI: 10.1111/j.1476-5381.2009.00229.x
Drug targets for traumatic brain injury from poly(ADP-ribose)polymerase pathway modulation
Abstract
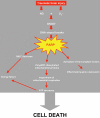
The deleterious pathophysiological cascade induced after traumatic brain injury (TBI) is initiated by an excitotoxic process triggered by excessive glutamate release. Activation of the glutamatergic N-methyl-D-aspartate receptor, by increasing calcium influx, activates nitric oxide (NO) synthases leading to a toxic production of NO. Moreover, after TBI, free radicals are highly produced and participate to a deleterious oxidative stress. Evidence has showed that the major toxic effect of NO comes from its combination with superoxide anion leading to peroxynitrite formation, a highly reactive and oxidant compound. Indeed, peroxynitrite mediates nitrosative stress and is a potent inducer of cell death through its reaction with lipids, proteins and DNA. Particularly DNA damage, caused by both oxidative and nitrosative stresses, results in activation of poly(ADP-ribose) polymerase (PARP), a nuclear enzyme implicated in DNA repair. In response to excessive DNA damage, massive PARP activation leads to energetic depletion and finally to cell death. Since 10 years, accumulating data have showed that inactivation of PARP, either pharmacologically or using PARP null mice, induces neuroprotection in experimental models of TBI. Thus TBI generating NO, oxidative and nitrosative stresses promotes PARP activation contributing in post-traumatic motor, cognitive and histological sequelae. The mechanisms by which PARP inhibitors provide protection might not entirely be related to the preservation of cellular energy stores, but might also include other PARP-mediated mechanisms that needed to be explored in a TBI context. Ten years of experimental research provided rational basis for the development of PARP inhibitors as treatment for TBI.
Figures

References
-
- Ahn MJ, Sherwood ER, Prough DS, Lin CY, De Witt DS. The effects of traumatic brain injury on cerebral blood flow and brain tissue nitric oxide levels and cytokine expression. J Neurotrauma. 2004;21:1431–1442. - PubMed
-
- Allen JW, Ivanova SA, Fan L, Espey MG, Basile AS, Faden AI. Group II metabotropic glutamate receptor activation attenuates traumatic neuronal injury and improves neurological recovery after traumatic brain injury. J Pharmacol Exp Ther. 1999;290:112–120. - PubMed
-
- Amé JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, et al. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–17868. - PubMed
-
- Ang BT, Yap E, Lim J, Tan WL, Ng PY, Ng I, et al. Poly(adenosine diphosphate-ribose) polymerase expression in human traumatic brain injury. J Neurosurg. 2003;99:125–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

