Effects of zotepine on extracellular levels of monoamine, GABA and glutamate in rat prefrontal cortex
- PMID: 19371334
- PMCID: PMC2707977
- DOI: 10.1111/j.1476-5381.2009.00175.x
Effects of zotepine on extracellular levels of monoamine, GABA and glutamate in rat prefrontal cortex
Abstract
Background and purpose: The atypical antipsychotic drug, zotepine, is effective in treatment of schizophrenia and acute mania, but the incidence of seizures during treatment is higher than with other antipsychotics. In addition, the mechanisms underlying the clinical actions of zotepine remain uncharacterized.
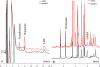
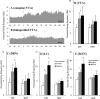
Experimental approach: The effects of intraperitoneal administration of zotepine and haloperidol on the extracellular levels of noradrenaline, dopamine, 5-HT, GABA, and glutamate in the medial prefrontal cortex (mPFC) were compared. Neuronal activities induced by each drug in the ventral tegmental area (VTA), locus coeruleus (LC), dorsal raphe nucleus (DRN) and mediodorsal thalamic nucleus (MTN) were also analysed.
Key results: Haloperidol did not affect extracellular neurotransmitter levels in the mPFC. In contrast, zotepine activated neuronal activities in all nuclei and increased the extracellular levels of noradrenaline, dopamine, GABA, and glutamate in the mPFC, but not 5-HT levels. The zotepine-stimulated neuronal activity in the VTA, LC, DRN and MTN enhanced the release of dopamine, noradrenaline, 5-HT, glutamate and GABA in the mPFC, although the enhanced GABAergic transmission possibly inhibited noradrenaline, dopamine and 5-HT release. The other afferent to mPFC, which releases dopamine and noradrenaline, was partially insensitive to GABAergic inhibition, but possibly received stimulatory AMPA/glutamatergic regulation from the MTN.
Conclusions and implications: Our results indicated that the positive interaction between prefrontal catecholaminergic transmission and AMPA/glutamatergic transmission from MTN might explain the regulatory effects of zotepine on neurotransmitter release. A mechanism is suggested to account for the pharmacological profile of this atypical antipsychotic and for its pro-convulsive action.
Figures

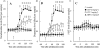
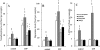
Similar articles
-
Effects of quetiapine on monoamine, GABA, and glutamate release in rat prefrontal cortex.Psychopharmacology (Berl). 2009 Oct;206(2):243-58. doi: 10.1007/s00213-009-1601-9. Epub 2009 Jul 3. Psychopharmacology (Berl). 2009. PMID: 19575183
-
Effect of novel atypical antipsychotic, blonanserin, on extracellular neurotransmitter level in rat prefrontal cortex.Eur J Pharmacol. 2011 Feb 25;653(1-3):47-57. doi: 10.1016/j.ejphar.2010.11.023. Epub 2010 Dec 11. Eur J Pharmacol. 2011. PMID: 21147094
-
In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain.Psychopharmacology (Berl). 2007 Apr;191(3):745-58. doi: 10.1007/s00213-007-0698-y. Epub 2007 Jan 30. Psychopharmacology (Berl). 2007. PMID: 17265076
-
Unraveling monoamine receptors involved in the action of typical and atypical antipsychotics on glutamatergic and serotonergic transmission in prefrontal cortex.Curr Pharm Des. 2010;16(5):502-15. doi: 10.2174/138161210790361416. Curr Pharm Des. 2010. PMID: 19909228 Review.
-
Glutamate co-transmission as an emerging concept in monoamine neuron function.J Psychiatry Neurosci. 2004 Jul;29(4):296-310. J Psychiatry Neurosci. 2004. PMID: 15309046 Free PMC article. Review.
Cited by
-
Pathogenesis and pathophysiology of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant α4 subunit of nicotinic ACh receptor.Br J Pharmacol. 2020 May;177(9):2143-2162. doi: 10.1111/bph.14974. Epub 2020 Feb 15. Br J Pharmacol. 2020. PMID: 31901135 Free PMC article.
-
Clozapine Normalizes a Glutamatergic Transmission Abnormality Induced by an Impaired NMDA Receptor in the Thalamocortical Pathway via the Activation of a Group III Metabotropic Glutamate Receptor.Biomolecules. 2019 Jun 17;9(6):234. doi: 10.3390/biom9060234. Biomolecules. 2019. PMID: 31213006 Free PMC article.
-
Thiamine tetrahydrofurfuryl disulfide promotes voluntary activity through dopaminergic activation in the medial prefrontal cortex.Sci Rep. 2018 Jul 11;8(1):10469. doi: 10.1038/s41598-018-28462-2. Sci Rep. 2018. PMID: 29992990 Free PMC article.
-
Antipsychotic treatment modulates glutamate transport and NMDA receptor expression.Eur Arch Psychiatry Clin Neurosci. 2014 Nov;264 Suppl 1:S67-82. doi: 10.1007/s00406-014-0534-4. Epub 2014 Sep 12. Eur Arch Psychiatry Clin Neurosci. 2014. PMID: 25214389 Review.
-
Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes.Br J Pharmacol. 2012 Mar;165(5):1543-55. doi: 10.1111/j.1476-5381.2011.01638.x. Br J Pharmacol. 2012. PMID: 21880034 Free PMC article.
References
-
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. - PubMed
-
- Aghajanian GK, Foote WE, Sheard MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970;171:178–187. - PubMed
-
- Aghajanian GK, Cedarbaum JM, Wang RY. Evidence for norepinephrine-mediated collateral inhibition of locus coeruleus neurons. Brain Res. 1977;136:570–577. - PubMed
-
- Amann B, Sterr A, Mergl R, Dittmann S, Seemuller F, Dobmeier M, et al. Zotepine loading in acute and severely manic patients: a pilot study. Bipolar Disord. 2005;7:471–476. - PubMed
-
- Bertram EH, Zhang D, Williamson JM. Multiple roles of midline dorsal thalamic nuclei in induction and spread of limbic seizures. Epilepsia. 2008;49:256–268. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

