Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain
- PMID: 19371344
- PMCID: PMC2707976
- DOI: 10.1111/j.1476-5381.2009.00184.x
Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain
Abstract
Background and purpose: Activation of cannabinoid (CB) receptors decreases nociceptive transmission in inflammatory or neuropathic pain states. However, the effects of CB receptor agonists in post-operative pain remain to be investigated. Here, we characterized the anti-allodynic effects of WIN 55,212-2 (WIN) in a rat model of post-operative pain.
Experimental approach: WIN 55,212-2 was characterized in radioligand binding and in vitro functional assays at rat and human CB(1) and CB(2) receptors. Analgesic activity and site(s) of action of WIN were assessed in the skin incision-induced post-operative pain model in rats; receptor specificity was investigated using selective CB(1) and CB(2) receptor antagonists.
Key results: WIN 55,212-2 exhibited non-selective affinity and agonist efficacy at human and rat CB(1) versus CB(2) receptors. Systemic administration of WIN decreased injury-induced mechanical allodynia and these effects were reversed by pretreatment with a CB(1) receptor antagonist, but not with a CB(2) receptor antagonist, given by systemic, intrathecal and supraspinal routes. In addition, peripheral administration of both CB(1) and CB(2) antagonists blocked systemic WIN-induced analgesic activity.
Conclusions and implications: Both CB(1) and CB(2) receptors were involved in the peripheral anti-allodynic effect of systemic WIN in a pre-clinical model of post-operative pain. In contrast, the centrally mediated anti-allodynic activity of systemic WIN is mostly due to the activation of CB(1) but not CB(2) receptors at both the spinal cord and brain levels. However, the increased potency of WIN following i.c.v. administration suggests that its main site of action is at CB(1) receptors in the brain.
Figures
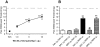
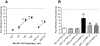
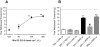

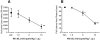
Similar articles
-
Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain.Brain Res. 2011 Sep 15;1412:44-54. doi: 10.1016/j.brainres.2011.07.031. Epub 2011 Jul 26. Brain Res. 2011. PMID: 21813113 Free PMC article.
-
Cannabinoid subtype-2 receptors modulate the antihyperalgesic effect of WIN 55,212-2 in rats with neuropathic spinal cord injury pain.Spine J. 2010 Dec;10(12):1049-54. doi: 10.1016/j.spinee.2010.08.015. Spine J. 2010. PMID: 20920894
-
Antinociceptive effect of intrathecal cannabinoid receptor agonist WIN 55,212-2 in a rat bone tumor pain model.Neurosci Lett. 2011 Apr 15;493(3):67-71. doi: 10.1016/j.neulet.2010.12.052. Epub 2010 Dec 31. Neurosci Lett. 2011. PMID: 21195743
-
Topical cannabinoid antinociception: synergy with spinal sites.Pain. 2003 Sep;105(1-2):11-6. doi: 10.1016/s0304-3959(03)00068-x. Pain. 2003. PMID: 14499415
-
Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain.Mol Neurobiol. 2007 Aug;36(1):26-35. doi: 10.1007/s12035-007-8007-7. Epub 2007 Oct 2. Mol Neurobiol. 2007. PMID: 17952647 Review.
Cited by
-
Local activation of cannabinoid CB₁ receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents.Mol Pain. 2011 May 9;7:31. doi: 10.1186/1744-8069-7-31. Mol Pain. 2011. PMID: 21554718 Free PMC article.
-
Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain.Brain Res. 2011 Sep 15;1412:44-54. doi: 10.1016/j.brainres.2011.07.031. Epub 2011 Jul 26. Brain Res. 2011. PMID: 21813113 Free PMC article.
-
Centrally mediated antinociceptive effects of cannabinoid receptor ligands in rat models of nociception.Pharmacol Biochem Behav. 2011 Dec;100(2):340-6. doi: 10.1016/j.pbb.2011.09.004. Epub 2011 Sep 17. Pharmacol Biochem Behav. 2011. PMID: 21958947 Free PMC article.
-
Activation of cannabinoid CB1 receptor contributes to suppression of spinal nociceptive transmission and inhibition of mechanical hypersensitivity by Aβ-fiber stimulation.Pain. 2016 Nov;157(11):2582-2593. doi: 10.1097/j.pain.0000000000000680. Pain. 2016. PMID: 27589093 Free PMC article.
-
Prevalence of Cannabinoid (CBD) Use in Orthopaedic Sports Medicine Patients.Orthop J Sports Med. 2022 Apr 5;10(4):23259671221087629. doi: 10.1177/23259671221087629. eCollection 2022 Apr. Orthop J Sports Med. 2022. PMID: 35400139 Free PMC article.
References
-
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur J Neurosci. 2003;17:2611–2618. - PubMed
-
- Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, et al. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. - PubMed
-
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. - PubMed
-
- Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiol Clin North America. 2005;23:1–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

