Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones
- PMID: 19371346
- PMCID: PMC2707984
- DOI: 10.1111/j.1476-5381.2009.00167.x
Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones
Abstract
Background and purpose: The transient receptor potential (TRP) channels, transient receptor potential melastatin-1 (TRPM8) and transient receptor potential ankyrin-1 (TRPA1), are expressed in subpopulations of sensory neurones and have been proposed to mediate innocuous and noxious cold sensation respectively. The aim of this study was to compare TRPM8 and TRPA1 modulation of glutamatergic afferent transmission within the spinal dorsal horn.
Experimental approach: Whole cell patch clamp recordings were made from rat spinal cord slices in vitro to examine the effect of TRP agonists and temperature on glutamatergic excitatory postsynaptic currents (EPSCs).
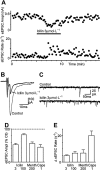
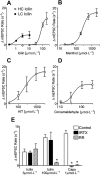
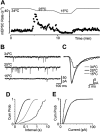
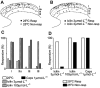
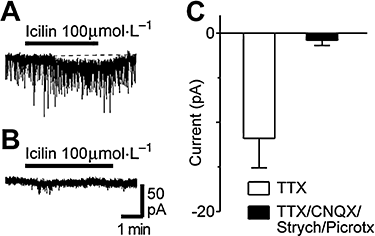
Key results: Icilin (3 or 100 micromol.L(-1)), menthol (200 micromol.L(-1)) and capsaicin (1 micromol.L(-1)) reduced the amplitude of primary afferent evoked EPSCs in subpopulations of lamina I and II neurones. In a subpopulation of superficial neurones, innocuous cold (threshold 29 degrees C), 3 micromol.L(-1) icilin (EC50 1.5 micromol.L(-1)) and menthol (EC50 263 micromol.L(-1)) increased the rate of spontaneous miniature EPSCs. In the majority of lamina I and II neurones, 100 micromol.L(-1) icilin (EC50 79 micromol.L(-1)), allyl isothiocyanate (EC50 226 micromol.L(-1)), cinnamaldehyde (EC50 38 micromol.L(-1)) and capsaicin (1 micromol.L(-1)) increased miniature EPSC rate. The response to 100 micromol.L(-1), but not 3 micromol.L(-1) icilin, was abolished by ruthenium red, while neither was affected by iodoresiniferatoxin. Responsiveness to 3 micromol.L(-1), but not to 100 micromol.L(-1) icilin, was highly predictive of innocuous cold responsiveness. Neurones responding to 3 micromol.L(-1) icilin and innocuous cold were located more superficially than those responding to 100 micromol.L(-1) icilin.
Conclusions and implications: Activation of TRPM8 and TRPA1 presynaptically modulated glutamatergic transmission onto partially overlapping but distinct populations of superficial dorsal horn neurones. Spinal TRPM8 and TRPA1 channels may therefore provide therapeutic targets in cold hyperesthesia.
Figures

Similar articles
-
TRPA1-like channels enhance glycinergic transmission in medullary dorsal horn neurons.J Neurochem. 2012 Aug;122(4):691-701. doi: 10.1111/j.1471-4159.2012.07817.x. Epub 2012 Jun 27. J Neurochem. 2012. PMID: 22671314
-
Comparison of icilin- and cold-evoked responses of spinal neurones, and their modulation of mechanical activity, in a model of neuropathic pain.Brain Res. 2008 Jun 18;1215:87-96. doi: 10.1016/j.brainres.2008.03.072. Epub 2008 Apr 9. Brain Res. 2008. PMID: 18479674
-
Dissociation of μ- and δ-opioid inhibition of glutamatergic synaptic transmission in superficial dorsal horn.Mol Pain. 2010 Oct 26;6:71. doi: 10.1186/1744-8069-6-71. Mol Pain. 2010. PMID: 20977770 Free PMC article.
-
Modulation of thermoreceptor TRPM8 by cooling compounds.ACS Chem Neurosci. 2012 Apr 18;3(4):248-67. doi: 10.1021/cn300006u. Epub 2012 Feb 13. ACS Chem Neurosci. 2012. PMID: 22860192 Free PMC article. Review.
-
How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation.Mol Pain. 2005 Apr 22;1:16. doi: 10.1186/1744-8069-1-16. Mol Pain. 2005. PMID: 15847696 Free PMC article. Review.
Cited by
-
Inhibitory Kcnip2 neurons of the spinal dorsal horn control behavioral sensitivity to environmental cold.Neuron. 2023 Jan 4;111(1):92-105.e5. doi: 10.1016/j.neuron.2022.10.008. Epub 2022 Nov 1. Neuron. 2023. PMID: 36323322 Free PMC article.
-
Spinal cord thermosensitivity: An afferent phenomenon?Temperature (Austin). 2016 Feb 26;3(2):232-239. doi: 10.1080/23328940.2016.1157665. eCollection 2016 Apr-Jun. Temperature (Austin). 2016. PMID: 27857953 Free PMC article. Review.
-
Endogenous transient receptor potential ankyrin 1 and vanilloid 1 activity potentiates glutamatergic input to spinal lamina I neurons in inflammatory pain.J Neurochem. 2019 May;149(3):381-398. doi: 10.1111/jnc.14677. Epub 2019 Mar 26. J Neurochem. 2019. PMID: 30716174 Free PMC article.
-
TRP Channels Involved in Spontaneous L-Glutamate Release Enhancement in the Adult Rat Spinal Substantia Gelatinosa.Cells. 2014 Apr 29;3(2):331-62. doi: 10.3390/cells3020331. Cells. 2014. PMID: 24785347 Free PMC article.
-
Role of transient receptor potential channels in cholecystokinin-induced activation of cultured vagal afferent neurons.Endocrinology. 2010 Nov;151(11):5237-46. doi: 10.1210/en.2010-0504. Epub 2010 Sep 29. Endocrinology. 2010. PMID: 20881249 Free PMC article.
References
-
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

