Neuropeptide S selectively inhibits the release of 5-HT and noradrenaline from mouse frontal cortex nerve endings
- PMID: 19371348
- PMCID: PMC2707993
- DOI: 10.1111/j.1476-5381.2009.00163.x
Neuropeptide S selectively inhibits the release of 5-HT and noradrenaline from mouse frontal cortex nerve endings
Abstract
Background and purpose: Neuropeptide S (NPS) is a recently identified neurotransmitter/neuromodulator able to increase arousal and wakefulness while decreasing anxiety-like behaviour. As several classical transmitters play a role in arousal and anxiety, we here investigated the possible presynaptic regulation of transmitter release by NPS.
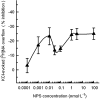
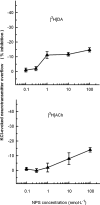
Experimental approach: Synaptosomes purified from mouse frontal cortex were prelabelled with [(3)H]5-hydroxytryptamine (5-HT), noradrenaline, dopamine, choline, D-aspartate or GABA and depolarized in superfusion with 12-15 mmol.L(-1) KCl to evoke [(3)H]neurotransmitter exocytosis. NPS was added at different concentrations (0.001 to 100 nmol.L(-1)).
Key results: NPS behaved as an extremely potent inhibitor of the evoked overflow of [(3)H]5-HT and [(3)H]noradrenaline exhibiting EC50 values in the low picomolar range. The inhibitory action of NPS on [(3)H]5-HT release was mimicked by [Ala(2)]NPS that was, however, about 100-fold less potent than the natural peptide. NPS (up to 100 nmol.L(-1)) was unable to affect the depolarization-evoked overflow of [(3)H]D-aspartate and [(3)H]GABA. The neuropeptide only weakly reduced the overflow of [(3)H]dopamine and [(3)H]ACh when added at relatively high concentrations.
Conclusions and implications: NPS, at low picomolar concentrations, can selectively inhibit the evoked release of 5-HT and noradrenaline in the frontal cortex by acting directly on 5-hydroxytryptaminergic and noradrenergic nerve terminals. These direct effects may explain only in part the unique behavioural activities of NPS, while an indirect involvement of other transmitters, especially of glutamate, must be considered.
Figures
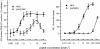
Similar articles
-
Neuropeptide S inhibits release of 5-HT and glycine in mouse amygdala and frontal/prefrontal cortex through activation of the neuropeptide S receptor.Neurochem Int. 2013 Mar;62(4):360-6. doi: 10.1016/j.neuint.2013.02.003. Epub 2013 Feb 11. Neurochem Int. 2013. PMID: 23411412
-
Effects of oxiracetam on neurotransmitter release from rat hippocampus slices and synaptosomes.Neurosci Lett. 1992 Sep 28;145(1):109-13. doi: 10.1016/0304-3940(92)90215-s. Neurosci Lett. 1992. PMID: 1361044
-
Differential desensitization of ionotropic non-NMDA receptors having distinct neuronal location and function.Naunyn Schmiedebergs Arch Pharmacol. 1997 Jul;356(1):29-38. doi: 10.1007/pl00005025. Naunyn Schmiedebergs Arch Pharmacol. 1997. PMID: 9228187
-
5-HT receptor regulation of neurotransmitter release.Pharmacol Rev. 2007 Dec;59(4):360-417. doi: 10.1124/pr.107.07103. Pharmacol Rev. 2007. PMID: 18160701 Review.
-
Amino acid transport in isolated neurons and glia.Adv Exp Med Biol. 1976;69:221-36. doi: 10.1007/978-1-4684-3264-0_17. Adv Exp Med Biol. 1976. PMID: 7926 Review.
Cited by
-
Intranasally administered neuropeptide S (NPS) exerts anxiolytic effects following internalization into NPS receptor-expressing neurons.Neuropsychopharmacology. 2012 May;37(6):1323-37. doi: 10.1038/npp.2011.317. Epub 2012 Jan 25. Neuropsychopharmacology. 2012. PMID: 22278093 Free PMC article.
-
Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala.Neuropsychopharmacology. 2012 Jun;37(7):1588-99. doi: 10.1038/npp.2012.3. Epub 2012 Feb 1. Neuropsychopharmacology. 2012. PMID: 22298122 Free PMC article.
-
Anxiety and Depression: What Do We Know of Neuropeptides?Behav Sci (Basel). 2022 Jul 29;12(8):262. doi: 10.3390/bs12080262. Behav Sci (Basel). 2022. PMID: 36004833 Free PMC article. Review.
-
Chronic treatment with escitalopram and venlafaxine affects the neuropeptide S pathway differently in adult Wistar rats exposed to maternal separation.AIMS Neurosci. 2022 Sep 13;9(3):395-422. doi: 10.3934/Neuroscience.2022022. eCollection 2022. AIMS Neurosci. 2022. PMID: 36329901 Free PMC article.
-
Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex.J Neurochem. 2010 Oct;115(2):475-82. doi: 10.1111/j.1471-4159.2010.06947.x. Epub 2010 Aug 30. J Neurochem. 2010. PMID: 20722970 Free PMC article.
References
-
- Amiel JM, Mathew SJ. Glutamate and anxiety disorders. Curr Psychiatry Rep. 2007;9:278–283. - PubMed
-
- Badia-Elder NE, Henderson AN, Bertholomey ML, Dodge NC, Stewart RB. The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2008;32:1380–1387. - PubMed
-
- Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem Biophys Res Commun. 2005;332:859–865. - PubMed
-
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. - PubMed
-
- Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

