Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats
- PMID: 19371585
- PMCID: PMC3820009
- DOI: 10.1016/j.neuropharm.2009.01.020
Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa-dependent abnormal involuntary movements in rats
Abstract
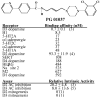
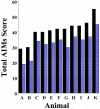
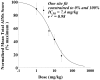
The D3 dopamine receptor selective antagonist PG01037 has been evaluated for the ability to attenuate L-dopa-associated abnormal involuntary movements (AIMs) in unilaterally lesioned male Sprague-Dawley rats, which is a model of L-dopa-dependent dyskinesia in patients with Parkinson's Disease. The intrinsic activity of PG01037 was determined using a) a forskolin-dependent adenylyl cyclase inhibition assay with transfected HEK 293 cells expressing either the human D2Long or D3 dopamine receptor subtype and b) an assay for agonist-associated mitogenesis. For the initial experiments, the 5-HT1A receptor selective partial agonist buspirone was used as a positive control to verify our ability to quantitate changes in total AIMs and AIMs minus locomotor scores. Subcutaneous (s.c.) administration of PG01037 was found to have minimal effect on AIMs score. However, it was observed that the in vivo efficacy of PG01037 increased when administered by intraperitoneal (i.p.) injection 15 min after L-dopa/benserazide administration, as compared to a 60 min, 30 min or 0 min pretreatment. It was also found that i.p. administration of PG01037 could inhibit involuntary movements after they had achieved maximum intensity. PG01037 was found to attenuate AIM scores in these animals in a dose dependent manner with IC(50) value equal to a) 7.4 mg/kg following L-dopa/benserazide administration (8 mg/kg each, i.p.) and b) 18.4 mg/kg following the administration of apomorphine (0.05 mg/kg, s.c.). However, PG01037 did not effectively inhibit SKF 81297-dependent abnormal involuntary movements. Rotarod studies indicate that PG01037 at a dose of 10 mg/kg did not adversely affect motor coordination of the unilaterally lesioned rats. Evaluation of lesioned rats using a cylinder test behavioral paradigm indicated that PG01037 did not dramatically attenuate the beneficial effects of L-dopa. These studies suggest that D3 dopamine receptor selective antagonists are potential pharmacotherapeutic candidates for the treatment of L-dopa-associated dyskinesia in patients with Parkinson's Disease.
Figures


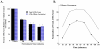






Similar articles
-
Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats.Neuropharmacology. 2011 Feb-Mar;60(2-3):284-94. doi: 10.1016/j.neuropharm.2010.09.011. Epub 2010 Sep 17. Neuropharmacology. 2011. PMID: 20850462 Free PMC article.
-
Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa-dependent abnormal involuntary movements in rats.Neuropharmacology. 2009 May-Jun;56(6-7):956-69. doi: 10.1016/j.neuropharm.2009.01.019. Epub 2009 Feb 5. Neuropharmacology. 2009. PMID: 19371586 Free PMC article.
-
The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats.Neuropharmacology. 2010 Feb;58(2):528-36. doi: 10.1016/j.neuropharm.2009.08.017. Epub 2009 Sep 4. Neuropharmacology. 2010. PMID: 19733554
-
Intranigral administration of substance P receptor antagonist attenuated levodopa-induced dyskinesia in a rat model of Parkinson's disease.Exp Neurol. 2015 Sep;271:168-74. doi: 10.1016/j.expneurol.2015.05.007. Epub 2015 May 20. Exp Neurol. 2015. PMID: 26001615
-
NLX-112, a novel 5-HT1A receptor agonist for the treatment of L-DOPA-induced dyskinesia: Behavioral and neurochemical profile in rat.Exp Neurol. 2015 Sep;271:335-50. doi: 10.1016/j.expneurol.2015.05.021. Epub 2015 May 30. Exp Neurol. 2015. PMID: 26037043
Cited by
-
Dopamine D3 receptors regulate reconsolidation of cocaine memory.Neuroscience. 2013 Jun 25;241:32-40. doi: 10.1016/j.neuroscience.2013.03.005. Epub 2013 Mar 16. Neuroscience. 2013. PMID: 23506736 Free PMC article.
-
Evaluation of Substituted N-Phenylpiperazine Analogs as D3 vs. D2 Dopamine Receptor Subtype Selective Ligands.Molecules. 2021 May 26;26(11):3182. doi: 10.3390/molecules26113182. Molecules. 2021. PMID: 34073405 Free PMC article.
-
Synthesis and in vitro pharmacological evaluation of indolyl carboxylic amide analogues as D3 dopamine receptor selective ligands.Medchemcomm. 2013 Sep;4(9):1283-1289. doi: 10.1039/C3MD00098B. Medchemcomm. 2013. PMID: 24156012 Free PMC article.
-
Characterization of the transport, metabolism, and pharmacokinetics of the dopamine D3 receptor-selective fluorenyl- and 2-pyridylphenyl amides developed for treatment of psychostimulant abuse.J Pharmacol Exp Ther. 2010 Jun;333(3):854-64. doi: 10.1124/jpet.109.165084. Epub 2010 Mar 12. J Pharmacol Exp Ther. 2010. PMID: 20228156 Free PMC article.
-
Evaluation of the D3 dopamine receptor selective agonist/partial agonist PG01042 on L-dopa dependent animal involuntary movements in rats.Neuropharmacology. 2011 Feb-Mar;60(2-3):284-94. doi: 10.1016/j.neuropharm.2010.09.011. Epub 2010 Sep 17. Neuropharmacology. 2011. PMID: 20850462 Free PMC article.
References
-
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levadopa-induced dyskinesia: Potential for new therapies. Nature Reviews Neuroscience. 2001;2:577–588. - PubMed
-
- Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature Medicine. 2003;9:762–767. - PubMed
-
- Blanchet PJ, Allard P, Gregoire L, Tardif F, Bédard PJ. Risk factors for peak dose dyskinesia in 100 levodopa-treated parkinsonian patients. Canadian J Neurological Science. 1996;23:189–193. - PubMed
-
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. European J Neuroscience. 2000;12:2117–2123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

