Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys
- PMID: 19371618
- PMCID: PMC2670960
- DOI: 10.1016/j.taap.2008.12.031
Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys
Abstract
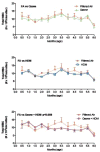
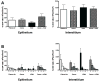
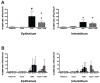
The epidemiologic link between air pollutant exposure and asthma has been supported by experimental findings, but the mechanisms are not understood. In this study, we evaluated the impact of combined ozone and house dust mite (HDM) exposure on the immunophenotype of peripheral blood and airway lymphocytes from rhesus macaque monkeys during the postnatal period of development. Starting at 30 days of age, monkeys were exposed to 11 cycles of filtered air, ozone, HDM aerosol, or ozone+HDM aerosol. Each cycle consisted of ozone delivered at 0.5 ppm for 5 days (8 h/day), followed by 9 days of filtered air; animals received HDM aerosol during the last 3 days of each ozone exposure period. Between 2-3 months of age, animals co-exposed to ozone+HDM exhibited a decline in total circulating leukocyte numbers and increased total circulating lymphocyte frequency. At 3 months of age, blood CD4+/CD25+ lymphocytes were increased with ozone+HDM. At 6 months of age, CD4+/CD25+ and CD8+/CD25+ lymphocyte populations increased in both blood and lavage of ozone+HDM animals. Overall volume of CD25+ cells within airway mucosa increased with HDM exposure. Ozone did not have an additive effect on volume of mucosal CD25+ cells in HDM-exposed animals, but did alter the anatomical distribution of this cell type throughout the proximal and distal airways. We conclude that a window of postnatal development is sensitive to air pollutant and allergen exposure, resulting in immunomodulation of peripheral blood and airway lymphocyte frequency and trafficking.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors of this paper declare that they have no conflicts of interest.
Figures




Similar articles
-
Organized lymphatic tissue (BALT) in lungs of rhesus monkeys after air pollutant exposure.Anat Rec (Hoboken). 2020 Nov;303(11):2766-2773. doi: 10.1002/ar.24456. Epub 2020 Jul 14. Anat Rec (Hoboken). 2020. PMID: 32445535 Free PMC article. Review.
-
Early life allergen and air pollutant exposures alter longitudinal blood immune profiles in infant rhesus monkeys.Toxicol Appl Pharmacol. 2017 Aug 1;328:60-69. doi: 10.1016/j.taap.2017.05.006. Epub 2017 May 18. Toxicol Appl Pharmacol. 2017. PMID: 28529118 Free PMC article.
-
Increased CCL24/eotaxin-2 with postnatal ozone exposure in allergen-sensitized infant monkeys is not associated with recruitment of eosinophils to airway mucosa.Toxicol Appl Pharmacol. 2011 Dec 15;257(3):309-18. doi: 10.1016/j.taap.2011.09.001. Epub 2011 Sep 12. Toxicol Appl Pharmacol. 2011. PMID: 21945493 Free PMC article.
-
Immune and airway effects of house dust mite aeroallergen exposures during postnatal development of the infant rhesus monkey.Clin Exp Allergy. 2003 Dec;33(12):1686-94. doi: 10.1111/j.1365-2222.2003.01812.x. Clin Exp Allergy. 2003. PMID: 14656356
-
The role of the house dust mite-induced innate immunity in development of allergic response.Int Arch Allergy Immunol. 2011;155(2):95-105. doi: 10.1159/000320375. Epub 2010 Dec 22. Int Arch Allergy Immunol. 2011. PMID: 21196753 Review.
Cited by
-
Organized lymphatic tissue (BALT) in lungs of rhesus monkeys after air pollutant exposure.Anat Rec (Hoboken). 2020 Nov;303(11):2766-2773. doi: 10.1002/ar.24456. Epub 2020 Jul 14. Anat Rec (Hoboken). 2020. PMID: 32445535 Free PMC article. Review.
-
Biochemical effects of ozone on asthma during postnatal development.Biochim Biophys Acta. 2011 Nov;1810(11):1114-9. doi: 10.1016/j.bbagen.2011.01.008. Epub 2011 Jan 27. Biochim Biophys Acta. 2011. PMID: 21276837 Free PMC article. Review.
-
Postnatal episodic ozone results in persistent attenuation of pulmonary and peripheral blood responses to LPS challenge.Am J Physiol Lung Cell Mol Physiol. 2011 Mar;300(3):L462-71. doi: 10.1152/ajplung.00254.2010. Epub 2010 Dec 3. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21131396 Free PMC article.
-
Early life allergen and air pollutant exposures alter longitudinal blood immune profiles in infant rhesus monkeys.Toxicol Appl Pharmacol. 2017 Aug 1;328:60-69. doi: 10.1016/j.taap.2017.05.006. Epub 2017 May 18. Toxicol Appl Pharmacol. 2017. PMID: 28529118 Free PMC article.
-
Early life exposure to allergen and ozone results in altered development in adolescent rhesus macaque lungs.Toxicol Appl Pharmacol. 2015 Feb 15;283(1):35-41. doi: 10.1016/j.taap.2014.12.006. Epub 2014 Dec 27. Toxicol Appl Pharmacol. 2015. PMID: 25545987 Free PMC article.
References
-
- Abel K, Alegria-Hartman MJ, Zanotto K, McChesney MB, Marthas ML, Miller CJ. Anatomic site and immune function correlate with relative cytokine mrna expression levels in lymphoid tissues of normal rhesus macaques. Cytokine. 2001;16:191–204. - PubMed
-
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus shiv89.6-induced protection against intravaginal challenge with pathogenic sivmac239 is independent of the route of immunization and is associated with a combination of cytotoxic t-lymphocyte and alpha interferon responses. J Virol. 2003;77:3099–3118. - PMC - PubMed
-
- Abel K, La Franco-Scheuch L, Rourke T, Ma ZM, De Silva V, Fallert B, Beckett L, Reinhart TA, Miller CJ. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus sivmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J Virol. 2004;78:841–854. - PMC - PubMed
-
- Antunez C, Torres MJ, Mayorga C, Corzo JL, Jurado A, SantamarÌa-Babi LF, Vera A, Blanca M. Cytokine production, activation marker, and skin homing receptor in children with atopic dermatitis and bronchial asthma. Pediatr Allergy Immunol. 2006;17:166–174. - PubMed
-
- Bottcher MF, Jenmalm MC, Voor T, Julge K, Holt PG, Bjorksten B. Cytokine responses to allergens during the first 2 years of life in estonian and swedish children. Clin Exp Allergy. 2006;36:619–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

