A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer
- PMID: 19371911
- PMCID: PMC4372725
- DOI: 10.1016/j.juro.2009.01.110
A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer
Abstract
Purpose: Most men with increased serum prostate specific antigen and negative biopsy require repeat biopsy because of the lack of a sensitive and specific prostate cancer detection test. In this study we evaluated the diagnostic potential of a duplex assay for prostate cancer by quantifying transcript levels of alpha-methylacyl-CoA racemase and prostate cancer antigen 3 in urine sediments following prostatic massage.
Materials and methods: Urine sediments from 92 patients, 43 with and 49 without prostate cancer, were collected after digital rectal examination. Transcript levels of AMACR, PCA3 and PSA in total RNA isolated from these samples were determined by absolute quantitative real-time polymerase chain reaction. AMACR and PCA3 scores were obtained by normalizing the transcript level to that of prostate specific antigen for each sample and multiplying by 100.
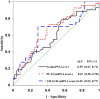
Results: AMACR (p = 0.006) and PCA3 (p = 0.014) scores, but not serum prostate specific antigen (p = 0.306), distinguished specimens from patients with prostate cancer from specimens from patients without prostate cancer, and ROC analysis established the diagnostic cutoff scores for the AMACR and PCA3 tests at 10.7 and 19.9, respectively. As determined from these cutoff scores the AMACR test had 70% (95% CI 56-83) sensitivity and 71% (95% CI 59-84) specificity, whereas the PCA3 test had 72% (95% CI 59-85) sensitivity and 59% (95% CI 45-73) specificity for prostate cancer detection. The combined use of AMACR and PCA3 scores in a dual marker test increased sensitivity to 81% (95% CI 70-93) and specificity to 84% (95% CI 73-94).
Conclusions: Urinary AMACR and PCA3 tests were superior to a serum prostate specific antigen test for detecting prostate cancer. Their combined use in a dual marker test further improved sensitivity and accuracy, and could serve as a surveillance test after repeat negative prostate biopsies.
Figures
Similar articles
-
Prostate Cancer Diagnosis Using Urine Sediment Analysis-Based α-Methylacyl-CoA Racemase Score: A Single-Center Experience.Cancer Control. 2019 Jan-Dec;26(1):1073274819887697. doi: 10.1177/1073274819887697. Cancer Control. 2019. PMID: 31793344 Free PMC article.
-
Detection of alpha-methylacyl-coenzyme-A racemase transcripts in blood and urine samples of prostate cancer patients.Mol Diagn Ther. 2006;10(6):397-403. doi: 10.1007/BF03256217. Mol Diagn Ther. 2006. PMID: 17154657
-
A novel diagnostic test for prostate cancer emerges from the determination of alpha-methylacyl-coenzyme a racemase in prostatic secretions.J Urol. 2004 Sep;172(3):1130-3. doi: 10.1097/01.ju.0000133560.87118.4d. J Urol. 2004. PMID: 15311056
-
The use of PCA3 in the diagnosis of prostate cancer.Nat Rev Urol. 2009 May;6(5):255-61. doi: 10.1038/nrurol.2009.40. Nat Rev Urol. 2009. PMID: 19424173 Review.
-
Urinary Prostate Cancer Antigen 3 as a Tumour Marker: Biochemical and Clinical Aspects.Adv Exp Med Biol. 2015;867:277-89. doi: 10.1007/978-94-017-7215-0_17. Adv Exp Med Biol. 2015. PMID: 26530372 Review.
Cited by
-
Diagnosis accuracy of PCA3 level in patients with prostate cancer: a systematic review with meta-analysis.Int Braz J Urol. 2020 Sep-Oct;46(5):691-704. doi: 10.1590/S1677-5538.IBJU.2019.0360. Int Braz J Urol. 2020. PMID: 31961625 Free PMC article.
-
Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro.PLoS One. 2014 Mar 3;9(3):e90332. doi: 10.1371/journal.pone.0090332. eCollection 2014. PLoS One. 2014. PMID: 24594937 Free PMC article.
-
The present and future of prostate cancer urine biomarkers.Int J Mol Sci. 2013 Jun 17;14(6):12620-49. doi: 10.3390/ijms140612620. Int J Mol Sci. 2013. PMID: 23774836 Free PMC article. Review.
-
Evaluation of Combined Quantification of PCA3 and AMACR Gene Expression for Molecular Diagnosis of Prostate Cancer in Moroccan Patients by RT-qPCR.Asian Pac J Cancer Prev. 2016 Dec 1;17(12):5229-5235. doi: 10.22034/APJCP.2016.17.12.5229. Asian Pac J Cancer Prev. 2016. PMID: 28125866 Free PMC article.
-
A-methylacyl-CoA racemase (AMACR) and prostate-cancer risk: a meta-analysis of 4,385 participants.PLoS One. 2013 Oct 9;8(10):e74386. doi: 10.1371/journal.pone.0074386. eCollection 2013. PLoS One. 2013. PMID: 24130666 Free PMC article.
References
-
- Etzioni R. Statistical issues in the evaluation of screening and early detection modalities. Urol Oncol. 2008;26:308. - PubMed
-
- Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297. - PubMed
-
- Jiang Z, Woda BA. Diagnostic utility of alpha-methylacyl CoA racemase (P504S) on prostate needle biopsy. Adv Anat Pathol. 2004;11:316. - PubMed
-
- Adley BP, Yang XJ. Application of alpha-methylacyl coenzyme A racemase immunohistochemistry in the diagnosis of prostate cancer: a review. Anal Quant Cytol Histol. 2006;28:1. - PubMed
-
- Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

