Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry
- PMID: 19372185
- PMCID: PMC2865473
- DOI: 10.1373/clinchem.2009.123935
Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry
Abstract
Background: Protein biomarker candidates from discovery proteomics must be quantitatively verified in patient samples before they can progress to clinical validation. Here we demonstrate that peptide immunoaffinity enrichment coupled with stable isotope dilution mass spectrometry (SISCAPA-MRM) can be used to configure assays with performance suitable for candidate biomarker verification. As proof of principle, we configured SISCAPA assays for troponin I (cTnI), an established biomarker of cardiac injury, and interleukin 33 (IL-33), an emerging immunological and cardiovascular marker for which robust immunoassays are currently not available.
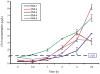
Methods: We configured individual and multiplexed assays in which peptides were enriched from digested human plasma using antipeptide antibodies. Assay performance was established using response curves for peptides and proteins spiked into normal plasma. We quantified proteins using labeled peptides as internal standards, and we measured levels of cTnI in patients who underwent a planned myocardial infarction for hypertrophic obstructive cardiomyopathy.
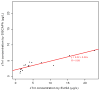
Results: Measurement of cTnI and IL-33 proteins from trypsin-digested plasma was linear from 1.5 to 5000 microg/L, with imprecision <13% for both proteins, processed individually or multiplexed. Results correlated well (R = 0.89) with a commercial immunoassay.
Conclusions: We used an established biomarker of cardiac injury and an emerging biomarker to demonstrate how SISCAPA can detect and quantify changes in concentration of proteins present at 1-10 microg/L in plasma. Our results demonstrate that these assays can be multiplexed and retain the necessary precision, reproducibility, and sensitivity to be applied to new and uncharacterized candidate biomarkers for verification of low-abundance proteins in blood.
Conflict of interest statement
Figures



References
-
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nature Biotechnol. 2006;24:971–83. - PubMed
-
- Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–88. - PubMed
-
- Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics. 2004;4:1175–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

