The role of calcium influx pathways in phospholipase D activation in bovine adrenal glomerulosa cells
- PMID: 19372190
- PMCID: PMC3743046
- DOI: 10.1677/JOE-09-0119
The role of calcium influx pathways in phospholipase D activation in bovine adrenal glomerulosa cells
Abstract
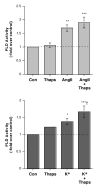
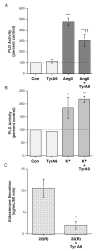
The steroid hormone aldosterone maintains sodium homeostasis and is therefore important in the control of blood volume and pressure. Angiotensin II (AngII) and elevated extracellular potassium concentrations ([K(+)](e)), the prime physiologic regulators of aldosterone secretion from adrenal glomerulosa cells, activate phospholipase D (PLD) in these cells. The role of Ca(2+) in the activation by these agents is unknown, although nitrendipine, a voltage-dependent Ca(2+) channel antagonist, does not inhibit AngII-elicited PLD activation, despite the fact that this compound blocked elevated [K(+)](e)-stimulated PLD activity. PLD activation triggered by AngII was also unaffected by the T-type calcium channel inhibitor nickel. Nevertheless, Ca(2+) influx was required for AngII-induced PLD activation in both primary cultures of bovine adrenal glomerulosa cells and a glomerulosa cell model, the NCI H295R adrenocortical carcinoma cell line. The involvement of store-operated Ca(2+) (SOC) influx and Ca(2+) release-activated Ca(2+) (CRAC) influx pathways in PLD activation was investigated using thapsigargin, an endoplasmic reticulum Ca(2+) pump inhibitor that empties the store to induce SOC influx, and the SOC inhibitor YM-58483 (BTP2), as well as a CRAC inhibitor, tyrphostin A9. In bovine glomerulosa cells, tyrphostin A9 inhibited AngII-induced PLD activation without affecting elevated [K(+)](e)-stimulated enzyme activity. On the other hand, differences were observed between the bovine adrenal glomerulosa and H295R cells in the involvement of Ca(2+) influx pathways in PLD activation, with the involvement of the SOC pathway suggested in the H295R cells. In summary, our results indicate that Ca(2+) entry only through certain Ca(2+) influx pathways is linked to PLD activation.
Figures







Similar articles
-
Mechanism of angiotensin II-induced phospholipase D activation in bovine adrenal glomerulosa cells.Mol Cell Endocrinol. 2002 Jun 28;192(1-2):7-16. doi: 10.1016/s0303-7207(02)00134-x. Mol Cell Endocrinol. 2002. PMID: 12088862
-
AngII induces transient phospholipase D activity in the H295R glomerulosa cell model.Mol Cell Endocrinol. 2003 Aug 29;206(1-2):113-22. doi: 10.1016/s0303-7207(03)00211-9. Mol Cell Endocrinol. 2003. PMID: 12943994
-
Characterization and phospholipase D mediation of the angiotensin II priming response in adrenal glomerulosa cells.Endocrinology. 2007 Feb;148(2):585-93. doi: 10.1210/en.2006-0898. Epub 2006 Nov 9. Endocrinology. 2007. PMID: 17095589
-
Control of aldosterone secretion: a model for convergence in cellular signaling pathways.Physiol Rev. 2004 Apr;84(2):489-539. doi: 10.1152/physrev.00030.2003. Physiol Rev. 2004. PMID: 15044681 Review.
-
Special features of mitochondrial Ca²⁺ signalling in adrenal glomerulosa cells.Pflugers Arch. 2012 Jul;464(1):43-50. doi: 10.1007/s00424-012-1086-y. Epub 2012 Mar 7. Pflugers Arch. 2012. PMID: 22395411 Review.
Cited by
-
LEFTY2 inhibits endometrial receptivity by downregulating Orai1 expression and store-operated Ca2+ entry.J Mol Med (Berl). 2018 Feb;96(2):173-182. doi: 10.1007/s00109-017-1610-9. Epub 2017 Dec 11. J Mol Med (Berl). 2018. PMID: 29230527 Free PMC article.
-
Anti-microbial cetylpyridinium chloride suppresses mast cell function by targeting tyrosine phosphorylation of Syk kinase.J Immunotoxicol. 2024 Dec;21(1):2443397. doi: 10.1080/1547691X.2024.2443397. Epub 2025 Jan 15. J Immunotoxicol. 2024. PMID: 39815634
-
NCI-H295R, a human adrenal cortex-derived cell line, expresses purinergic receptors linked to Ca²⁺-mobilization/influx and cortisol secretion.PLoS One. 2013 Aug 8;8(8):e71022. doi: 10.1371/journal.pone.0071022. eCollection 2013. PLoS One. 2013. PMID: 23951072 Free PMC article.
-
Antimicrobial agent triclosan suppresses mast cell signaling via phospholipase D inhibition.J Appl Toxicol. 2019 Dec;39(12):1672-1690. doi: 10.1002/jat.3884. Epub 2019 Aug 19. J Appl Toxicol. 2019. PMID: 31429102 Free PMC article.
References
-
- Aptel HB, Burnay MM, Rossier MF, Capponi AM. The role of tyrosine kinases in capacitative calcium influx-mediated aldosterone production in bovine adrenal zona glomerulosa cells. J Endocrinol. 1999;163(1):131–138. - PubMed
-
- Barrett PQ, Bollag WB, Isales CM, McCarthy RT, Rasmussen H. Role of calcium in angiotensin II-mediated aldosterone secretion. Endocr Rev. 1989;10(4):1–22. - PubMed
-
- Barrett PQ, Ertel EA, Smith MM, Nee JJ, Cohen CJ. Voltage-gated calcium currents have two opposing effects on the secretion of aldosterone. Am. J. Physiol. 1995;268:C985–C992. - PubMed
-
- Betancourt-Calle S, Jung EM, White S, Calle RA, Rasmussen H, Bollag WB. Elevated K + induces MARCKS phosphorylation and phospholipase D activation in glomerulosa cells. Mol. Cell. Endocrinol. 2001;184:65–76. - PubMed
-
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: A model for angiotensin-II-responsive aldosterone secretion. Endocrinology. 1993;133:1555–1561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

