Thiazolidinediones mimic glucose starvation in facilitating Sp1 degradation through the up-regulation of beta-transducin repeat-containing protein
- PMID: 19372209
- PMCID: PMC2701453
- DOI: 10.1124/mol.109.055376
Thiazolidinediones mimic glucose starvation in facilitating Sp1 degradation through the up-regulation of beta-transducin repeat-containing protein
Abstract
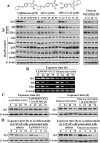
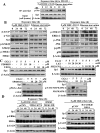
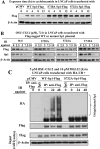
This study investigated the mechanism by which the transcription factor Sp1 is degraded in prostate cancer cells. We recently developed a thiazolidinedione derivative, (Z)-5-(4-hydroxy-3-trifluoromethylbenzylidene)-3-(1-methylcyclohexyl)-thiazolidine-2,4-dione (OSU-CG12), that induces Sp1 degradation in a manner paralleling that of glucose starvation. Based on our finding that thiazolidinediones suppress beta-catenin and cyclin D1 by up-regulating the E3 ligase SCF(beta-TrCP), we hypothesized that beta-transducin repeat-containing protein (beta-TrCP) targets Sp1 for proteasomal degradation in response to glucose starvation or OSU-CG12. Here we show that either treatment of LNCaP cells increased specific binding of Sp1 with beta-TrCP. This direct binding was confirmed by in vitro pull-down analysis with bacterially expressed beta-TrCP. Although ectopic expression of beta-TrCP enhanced the ability of OSU-CG12 to facilitate Sp1 degradation, suppression of endogenous beta-TrCP function by a dominant-negative mutant or small interfering RNA-mediated knockdown blocked OSU-CG12-facilitated Sp1 ubiquitination and/or degradation. Sp1 contains a C-terminal conventional DSG destruction box ((727)DSGAGS(732)) that mediates beta-TrCP recognition and encompasses a glycogen synthase kinase 3beta (GSK3beta) phosphorylation motif (SXXXS). Pharmacological and molecular genetic approaches and mutational analyses indicate that extracellular signal-regulated kinase-mediated phosphorylation of Thr739 and GSK3beta-mediated phosphorylation of Ser728 and Ser732 were critical for Sp1 degradation. The ability of OSU-CG12 to mimic glucose starvation to activate beta-TrCP-mediated Sp1 degradation has translational potential to foster novel strategies for cancer therapy.
Figures





References
-
- Benasciutti E, Pagès G, Kenzior O, Folk W, Blasi F, and Crippa MP (2004) MAPK and JNK transduction pathways can phosphorylate Sp1 to activate the uPA minimal promoter element and endogenous gene transcription. Blood 104 256-262. - PubMed
-
- Bhatia N, Thiyagarajan S, Elcheva I, Saleem M, Dlugosz A, Mukhtar H, and Spiegelman VS (2006) Gli2 is targeted for ubiquitination and degradation by beta-TrCP ubiquitin ligase. J Biol Chem 281 19320-19326. - PubMed
-
- Bonello MR and Khachigian LM (2004) Fibroblast growth factor-2 represses platelet-derived growth factor receptor-α (PDGFR-α) transcription via ERK1/2-dependent Sp1 phosphorylation and an atypical cis-acting element in the proximal PDGFR-α promoter. J Biol Chem 279 2377-2382. - PubMed
-
- Chu S and Ferro TJ (2006) Identification of a hydrogen peroxide-induced PP1-JNK1-Sp1 signaling pathway for gene regulation. Am J Physiol Lung Cell Mol Physiol 291 L983-L992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

