Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5
- PMID: 19372219
- PMCID: PMC2713507
- DOI: 10.1074/jbc.M109.008078
Mdm2 directs the ubiquitination of beta-arrestin-sequestered cAMP phosphodiesterase-4D5
Erratum in
- J Biol Chem. 2009 Aug 7;284(32):21776
Retraction in
-
Withdrawal: Mdm2 directs the ubiquitination of β-arrestin-sequestered cAMP phosphodiesterase-4D5.J Biol Chem. 2020 Aug 21;295(34):12328. doi: 10.1074/jbc.W120.015374. J Biol Chem. 2020. PMID: 32826336 Free PMC article. No abstract available.
Abstract
Beta-arrestin plays a key role in regulating beta2-adrenoreceptor signaling by interdicting activation of adenylyl cyclase and selectively sequestering cAMP phosphodiesterase-4D5 (PDE4D5) for delivery of an active cAMP degrading system to the site of cAMP synthesis. Here we show that the beta-agonist, isoprenaline, triggers the rapid and transient ubiquitination of PDE4D5 in primary cardiomyocytes, mouse embryo fibroblasts, and HEK293B2 cells constitutively expressing beta2-adrenoceptors. Reconstitution analyses in beta-arrestin1/2 double knockout cells plus small interference RNA knockdown studies indicate that a beta-arrestin-scaffolded pool of the E3-ubiquitin ligase, Mdm2, mediates PDE4D5 ubiquitination. Critical for this is the ubiquitin-interacting motif located in the extreme C terminus of PDE4D5, which is specific to the PDE4D sub-family. In vitro ubiquitination [corrected] of a PDE4D5 spot-immobilized peptide array, followed by a mutagenesis strategy, showed that PDE4D5 ubiquitination occurs at Lys-48, Lys-53, and Lys-78, which are located within its isoform-specific N-terminal region, as well as at Lys-140 located within its regulatory UCR1 module. We suggest that mono-ubiquitination at Lys-140 primes PDE4D5 for a subsequent cascade of polyubiquitination occurring within its isoform-specific N-terminal region at Lys-48, Lys-53, and Lys-78. PDE4D5 interacts with a non-ubiquitinated beta-arrestin sub-population that is likely to be protected from Mdm2-mediated ubiquitination due to steric hindrance caused by sequestered PDE4D5. Ubiquitination of PDE4D5 elicits an increase in the fraction of PDE4D5 sequestered by beta-arrestin in cells, thereby contributing to the fidelity of PDE4D5-beta-arrestin interaction, as well as decreasing the fraction of PDE4D5 sequestered by the scaffolding protein, RACK1.
Figures



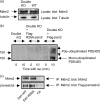




Similar articles
-
1H NMR structural and functional characterisation of a cAMP-specific phosphodiesterase-4D5 (PDE4D5) N-terminal region peptide that disrupts PDE4D5 interaction with the signalling scaffold proteins, beta-arrestin and RACK1.Cell Signal. 2007 Dec;19(12):2612-24. doi: 10.1016/j.cellsig.2007.08.015. Epub 2007 Sep 1. Cell Signal. 2007. PMID: 17900862
-
Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5.Biochem J. 2006 Aug 15;398(1):23-36. doi: 10.1042/BJ20060423. Biochem J. 2006. PMID: 16689683 Free PMC article.
-
RNA silencing identifies PDE4D5 as the functionally relevant cAMP phosphodiesterase interacting with beta arrestin to control the protein kinase A/AKAP79-mediated switching of the beta2-adrenergic receptor to activation of ERK in HEK293B2 cells.J Biol Chem. 2005 Sep 30;280(39):33178-89. doi: 10.1074/jbc.M414316200. Epub 2005 Jul 19. J Biol Chem. 2005. Retraction in: J Biol Chem. 2025 Mar;301(3):108141. doi: 10.1016/j.jbc.2024.108141. PMID: 16030021 Retracted.
-
cAMP-specific phosphodiesterase-4D5 (PDE4D5) provides a paradigm for understanding the unique non-redundant roles that PDE4 isoforms play in shaping compartmentalized cAMP cell signalling.Biochem Soc Trans. 2007 Nov;35(Pt 5):938-41. doi: 10.1042/BST0350938. Biochem Soc Trans. 2007. PMID: 17956250 Review.
-
The cyclic AMP phosphodiesterase 4D5 (PDE4D5)/receptor for activated C-kinase 1 (RACK1) signalling complex as a sensor of the extracellular nano-environment.Cell Signal. 2017 Jul;35:282-289. doi: 10.1016/j.cellsig.2017.01.013. Epub 2017 Jan 6. Cell Signal. 2017. PMID: 28069443 Review.
Cited by
-
Modulation of Compartmentalised Cyclic Nucleotide Signalling via Local Inhibition of Phosphodiesterase Activity.Int J Mol Sci. 2016 Oct 2;17(10):1672. doi: 10.3390/ijms17101672. Int J Mol Sci. 2016. PMID: 27706091 Free PMC article. Review.
-
Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways.Dev Cell. 2009 Oct;17(4):443-58. doi: 10.1016/j.devcel.2009.09.011. Dev Cell. 2009. PMID: 19853559 Free PMC article. Review.
-
Heat shock protein 20 (HSP20) is a novel substrate for protein kinase D1 (PKD1).Cell Biochem Funct. 2015 Oct;33(7):421-6. doi: 10.1002/cbf.3147. Epub 2015 Oct 6. Cell Biochem Funct. 2015. PMID: 26443497 Free PMC article.
-
Repeated shock stress facilitates basolateral amygdala synaptic plasticity through decreased cAMP-specific phosphodiesterase type IV (PDE4) expression.Brain Struct Funct. 2018 May;223(4):1731-1745. doi: 10.1007/s00429-017-1575-z. Epub 2017 Dec 4. Brain Struct Funct. 2018. PMID: 29204911 Free PMC article.
-
β-Arrestin-2 desensitizes the transient receptor potential vanilloid 1 (TRPV1) channel.J Biol Chem. 2012 Oct 26;287(44):37552-63. doi: 10.1074/jbc.M112.391847. Epub 2012 Sep 5. J Biol Chem. 2012. PMID: 22952227 Free PMC article.
References
-
- Beavo J. A., Brunton L. L. ( 2002) Nat. Rev. Mol. Cell Biol. 3, 710– 718 - PubMed
-
- Taskén K., Aandahl E. M. ( 2004) Physiol. Rev. 84, 137– 167 - PubMed
-
- Taylor S. S., Kim C., Vigil D., Haste N. M., Yang J., Wu J., Anand G. S. ( 2005) Biochim. Biophys. Acta 1754, 25– 37 - PubMed
-
- Wong W., Scott J. D. ( 2004) Nat. Rev. Mol. Cell Biol. 5, 959– 970 - PubMed
-
- Conti M., Beavo J. ( 2007) Annu. Rev. Biochem. 76, 481– 511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

