Endogenous betaglycan is essential for high-potency inhibin antagonism in gonadotropes
- PMID: 19372236
- PMCID: PMC2703594
- DOI: 10.1210/me.2009-0021
Endogenous betaglycan is essential for high-potency inhibin antagonism in gonadotropes
Abstract
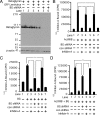
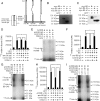
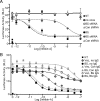
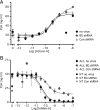
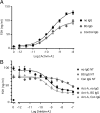
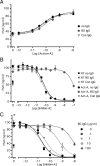
Inhibins are endocrine hormones that regulate gametogenesis and reproduction through a negative feedback loop with FSH. Inhibin action involves antagonism of signaling by activin or other TGFbeta family ligands. In transfection assays, antagonism by inhibin can be potentiated by betaglycan, a coreceptor for selected TGFbeta family ligands. We tested whether betaglycan is an obligate inhibin coreceptor through disruption of betaglycan function by RNA interference-mediated knockdown and immunoneutralization. Betaglycan knockdown and anti-betaglycan IgG each independently prevented inhibin-A binding to betaglycan and reversed functional effects of transfected betaglycan. Neither betaglycan immunoneutralization nor knockdown affected activin responsiveness in cell lines or in rat anterior pituitary cultures. Betaglycan knockdown decreased the potency of inhibin antagonism of activin-induced FSH secretion in primary gonadotropes. Similarly, anti-betaglycan IgG decreased the potency of inhibin antagonism in primary gonadotropes in a dose-dependent manner, with a reduction in the sensitivity to inhibin-A of greater than 1000-fold. These data establish that betaglycan is an endogenous inhibin coreceptor required for high-sensitivity inhibin antagonism of activin signaling in rat anterior pituitary gonadotropes.
Figures
Similar articles
-
Reducing betaglycan expression by RNA interference (RNAi) attenuates inhibin bioactivity in LbetaT2 gonadotropes.Mol Cell Endocrinol. 2009 Aug 13;307(1-2):149-56. doi: 10.1016/j.mce.2009.03.021. Epub 2009 Apr 8. Mol Cell Endocrinol. 2009. PMID: 19524135
-
Betaglycan localization in the female rat pituitary: implications for the regulation of follicle-stimulating hormone by inhibin.Endocrinology. 2003 Dec;144(12):5640-9. doi: 10.1210/en.2003-0670. Epub 2003 Sep 18. Endocrinology. 2003. PMID: 14500575
-
Transforming growth factor-beta modulates inhibin A bioactivity in the LbetaT2 gonadotrope cell line by competing for binding to betaglycan.Mol Endocrinol. 2002 Dec;16(12):2754-63. doi: 10.1210/me.2002-0014. Mol Endocrinol. 2002. PMID: 12456797
-
A Tale of Two Proteins: Betaglycan, IGSF1, and the Continuing Search for the Inhibin B Receptor.Trends Endocrinol Metab. 2020 Jan;31(1):37-45. doi: 10.1016/j.tem.2019.08.014. Epub 2019 Oct 22. Trends Endocrinol Metab. 2020. PMID: 31648935 Review.
-
Molecular biology of inhibin action.Semin Reprod Med. 2004 Aug;22(3):269-76. doi: 10.1055/s-2004-831902. Semin Reprod Med. 2004. PMID: 15319829 Review.
Cited by
-
Serum inhibin A and inhibin B levels in epithelial ovarian cancer patients.PLoS One. 2014 Mar 5;9(3):e90575. doi: 10.1371/journal.pone.0090575. eCollection 2014. PLoS One. 2014. PMID: 24599287 Free PMC article.
-
From Consternation to Revelation: Discovery of a Role for IGSF1 in Pituitary Control of Thyroid Function.J Endocr Soc. 2018 Feb 6;2(3):220-231. doi: 10.1210/js.2017-00478. eCollection 2018 Mar 1. J Endocr Soc. 2018. PMID: 29594256 Free PMC article. Review.
-
The Local Control of the Pituitary by Activin Signaling and Modulation.Open Neuroendocrinol J. 2011 Jan 1;4:90-101. doi: 10.2174/1876528901104010090. Open Neuroendocrinol J. 2011. PMID: 21927629 Free PMC article.
-
Cell-type specific modulation of pituitary cells by activin, inhibin and follistatin.Mol Cell Endocrinol. 2012 Aug 15;359(1-2):43-52. doi: 10.1016/j.mce.2012.01.025. Epub 2012 Feb 4. Mol Cell Endocrinol. 2012. PMID: 22330643 Free PMC article. Review.
-
TGFBR3L is an inhibin B co-receptor that regulates female fertility.Sci Adv. 2021 Dec 17;7(51):eabl4391. doi: 10.1126/sciadv.abl4391. Epub 2021 Dec 15. Sci Adv. 2021. PMID: 34910520 Free PMC article.
References
-
- Vale W, Bilezikjian LM, Rivier C 1994 Reproductive and other roles of inhibins and activins. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven Press; 1861–1877
-
- Wiater E, Vale W 2008 Activins and inhibins. In: Derynck R, Miyazono K, eds. The biology of the TGF-β family. New York: Cold Spring Harbor Laboratory Press; 79–120
-
- Woodruff TK, Mather JP 1995 Inhibin, activin and the female reproductive axis. Annu Rev Physiol 57:219–244 - PubMed
-
- Cook RW, Thompson TB, Jardetzky TS, Woodruff TK 2004 Molecular biology of inhibin action. Semin Reprod Med 22:269–276 - PubMed
-
- Rivier C, Vale W 1989 Immunoneutralization of endogenous inhibin modifies hormone secretion and ovulation rate in the rat. Endocrinology 125:152–157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

