How I treat paroxysmal nocturnal hemoglobinuria
- PMID: 19372253
- PMCID: PMC2710914
- DOI: 10.1182/blood-2009-03-195966
How I treat paroxysmal nocturnal hemoglobinuria
Abstract
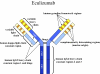
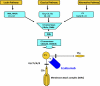
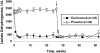
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare clonal blood disorder that manifests with hemolytic anemia, bone marrow failure, and thrombosis. Many of the clinical manifestations of the disease result from complement-mediated intravascular hemolysis. Allogeneic bone marrow transplantation is the only curative therapy for PNH. Eculizumab, a monoclonal antibody that blocks terminal complement activation, is highly effective in reducing hemolysis, improving quality of life, and reducing the risk for thrombosis in PNH patients. Insights into the relevance of detecting PNH cells in PNH and other bone marrow failure disorders are highlighted, and indications for treating PNH patients with bone marrow transplantation and eculizumab are explored.
Figures

References
-
- Brodsky RA. Narrative review. Paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148:587–595. - PubMed
-
- Parker CJ. Historical aspects of paroxysmal nocturnal haemoglobinuria: “defining the disease.”. Br J Haematol. 2002;117:3–22. - PubMed
-
- Miyata T, Takeda J, Iida Y, et al. The cloning of PIG-A, a component in the early step of GPI-anchor biosynthesis. Science. 1993;259:1318–1320. - PubMed
-
- Miyata T, Yamada N, Iida Y, et al. Abnormalities of PIG-A transcripts in granulocytes from patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1994;330:249–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

