Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy
- PMID: 19372302
- PMCID: PMC2692772
- DOI: 10.1152/japplphysiol.00295.2009
Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy
Abstract
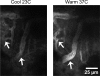
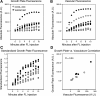
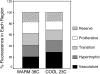
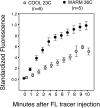
Solute delivery to avascular cartilaginous plates is critical to bone elongation, and impaired transport of nutrients and growth factors in cartilage matrix could underlie many skeletal abnormalities. Advances in imaging technology have revolutionized our ability to visualize growth plates in vivo, but quantitative methods are still needed. We developed analytical standards for measuring solute delivery, defined by amount and rate of intravenous tracer entry, in murine growth plates using multiphoton microscopy. We employed an acute temperature model because of its well-established impact on bone circulation and tested the hypothesis that solute delivery changes positively with limb temperature when body core and respiration are held constant (36 degrees C, 120 breaths/min). Tibial growth plates were surgically exposed in anesthetized 5-wk-old mice, and their hindlimbs were immersed in warm (36 degrees C) or cool (23 degrees C) saline (n = 6/group). After 30 min of thermal equilibration, we administered an intracardiac injection of fluorescein (50 microl, 0.5%) and captured sequentially timed growth plate images spanning 10 min at standardized depth. Absolute growth plate fluorescence was normalized to vascular concentrations for interanimal comparisons. As predicted, more fluorescein infiltrated growth plates at 36 degrees C, with standardized values nearly double those at 23 degrees C. Changing initial limb temperature did not alter baseline values, suggesting a sustained response period. These data validate the sensitivity of our system and have relevance to strategies for enhancing localized delivery of therapeutic agents to growth plates of children. Applications of this technique include assessment of solute transport in models of growth plate dysfunction, particularly chondrodysplasias with matrix irregularities.
Figures




References
-
- Aigner T, Rau T, Niederhagen M, Zaucke F, Schmitz M, Pöhls U, Stöss H, Rauch A, Thiel CT. Achondrogenesis type IA (Houston-Harris): a still unresolved molecular phenotype. Pediatr Dev Pathol 10: 328–334, 2007. - PubMed
-
- Akorn. AK-FLUOR Fluorescein Injection, USP (Online). http://www.akorn.com/documents/catalog/sell_sheets/17478-253-10.pdf?PHPS... [2009].
-
- Alman BA Skeletal dysplasias and the growth plate. Clin Genet 73: 24–30, 2008. - PubMed
-
- Ang C, Dawes J. The effects of hyperthermia on human endothelial monolayers: modulation of thrombotic potential and permeability. Blood Coagul Fibrinolysis 5: 193–199, 1994. - PubMed
-
- Arkill KP, Winlove CP. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage 16: 708–714, 2008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

