Neuritogenic actions of botulinum neurotoxin A on cultured motor neurons
- PMID: 19372387
- PMCID: PMC2700173
- DOI: 10.1124/jpet.108.147744
Neuritogenic actions of botulinum neurotoxin A on cultured motor neurons
Abstract
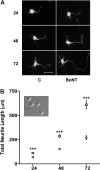
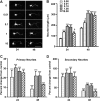
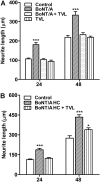
Botulinum neurotoxins (BoNTs) are extremely potent neuromuscular poisons that act through soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein cleavage to inhibit neurotransmitter release. The ability of BoNT serotype A (BoNT/A) to eliminate localized transmitter release at extremely low doses is well characterized. In the current study, we investigated the less understood characteristic of BoNT/A to induce nerve outgrowth, sometimes referred to as sprouting. This phenomenon is generally considered a secondary response to the paralytic actions of BoNT/A, and other potential factors that may initiate this sprouting have not been investigated. Alternatively, we hypothesized that BoNT/A induces sprouting through presynaptic receptor activation that is independent of its known intracellular actions on the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) synaptosomal associated protein of 25 kDa (SNAP-25). To test this, the effects of BoNT/A application on neurite outgrowth were examined using primary cultures enriched with motor neurons isolated from embryonic mouse spinal cord. In this system, BoNT/A potently stimulated neuritogenesis at concentrations as low as 0.01 nM. The neuritogenic effects of BoNT/A exposure were concentration dependent and antagonized by Triticum vulgaris lectin, a known competitive antagonist of BoNT. Similar results were observed with the isolated BoNT/A binding domain, revealing that neuritogenesis could be initiated solely by the binding actions of BoNT/A. In addition, the presence or absence of SNAP-25 cleavage by BoNT/A was not a determinant factor in BoNT/A-induced neuritogenesis. Collectively, these results suggest that binding of BoNT/A to the motor neuronal membrane activates neuritogenesis through as yet undetermined intracellular pathway(s), independent of its known action on vesicular release.
Figures
Similar articles
-
Fusion of Golgi-derived vesicles mediated by SNAP-25 is essential for sympathetic neuron outgrowth but relatively insensitive to botulinum neurotoxins in vitro.FEBS J. 2014 Jul;281(14):3243-60. doi: 10.1111/febs.12858. Epub 2014 Jun 13. FEBS J. 2014. PMID: 24863955
-
Uptake of botulinum neurotoxin into cultured neurons.Biochemistry. 2004 Jan 20;43(2):526-32. doi: 10.1021/bi0356698. Biochemistry. 2004. PMID: 14717608
-
Syntaxin and 25-kDa synaptosomal-associated protein: differential effects of botulinum neurotoxins C1 and A on neuronal survival.J Neurosci Res. 1998 Jun 1;52(5):569-83. doi: 10.1002/(SICI)1097-4547(19980601)52:5<569::AID-JNR9>3.0.CO;2-A. J Neurosci Res. 1998. PMID: 9632313
-
Botulinum neurotoxin type A: Actions beyond SNAP-25?Toxicology. 2015 Sep 1;335:79-84. doi: 10.1016/j.tox.2015.07.003. Epub 2015 Jul 10. Toxicology. 2015. PMID: 26169827 Review.
-
Botulinum toxin type A in motor nervous system: unexplained observations and new challenges.J Neural Transm (Vienna). 2016 Dec;123(12):1415-1421. doi: 10.1007/s00702-016-1611-9. Epub 2016 Sep 1. J Neural Transm (Vienna). 2016. PMID: 27586162 Review.
Cited by
-
Neurotrophic effects of Botulinum neurotoxin type A in hippocampal neurons involve activation of Rac1 by the non-catalytic heavy chain (HCC/A).IBRO Neurosci Rep. 2021 May 13;10:196-207. doi: 10.1016/j.ibneur.2021.04.002. eCollection 2021 Jun. IBRO Neurosci Rep. 2021. PMID: 34041508 Free PMC article.
-
Restoration of Memory Along With Neurogenic Enhancement by Therapeutic Botulinum Neurotoxin in a Preclinical Model of Parkinson's Disease.Am J Alzheimers Dis Other Demen. 2025 Jan-Dec;40:15333175251346292. doi: 10.1177/15333175251346292. Epub 2025 May 30. Am J Alzheimers Dis Other Demen. 2025. PMID: 40444534 Free PMC article.
-
Therapeutic use of botulinum toxin in pain treatment.Neuronal Signal. 2018 Aug 31;2(3):NS20180058. doi: 10.1042/NS20180058. eCollection 2018 Sep. Neuronal Signal. 2018. PMID: 32714587 Free PMC article. Review.
-
The Effect of Botulinum Neurotoxin Serotype a Heavy Chain on the Growth Related Proteins and Neurite Outgrowth after Spinal Cord Injury in Rats.Toxins (Basel). 2018 Feb 2;10(2):66. doi: 10.3390/toxins10020066. Toxins (Basel). 2018. PMID: 29393906 Free PMC article.
-
Effects of botulinum toxin A on endometriosis‑associated pain and its related mechanism.Mol Med Rep. 2020 Nov;22(5):4351-4359. doi: 10.3892/mmr.2020.11501. Epub 2020 Sep 10. Mol Med Rep. 2020. Retraction in: Mol Med Rep. 2021 Jan;23(1):36. doi: 10.3892/mmr.2020.11674. PMID: 33000241 Free PMC article. Retracted.
References
-
- Ahnert-Hilger G, Höltje M, Grosse G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, and Just I (2004) Differential effects of rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem 90 9-18. - PubMed
-
- Anderson KN, Potter AC, Piccenna LG, Quah AK, Davies KE, and Cheema SS (2004) Isolation and culture of motor neurons from the newborn mouse spinal cord. Brain Res Brain Res Protoc 12 132-136. - PubMed
-
- Angaut-Petit D, Molgó J, Comella JX, Faille L, and Tabti N (1990) Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience 37 799-808. - PubMed
-
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al. (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA 285 1059-1070. - PubMed
-
- Bakry N, Kamata Y, and Simpson LL (1991) Lectins from Triticum vulgaris and Limax flavus are universal antagonists of botulinum neurotoxin and tetanus toxin. J Pharmacol Exp Ther 258 830-836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

