A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers
- PMID: 19372436
- PMCID: PMC2679824
- DOI: 10.1503/cmaj.090523
A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers
Figures
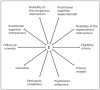
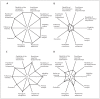
Comment in
-
Explaining pragmatic trials to pragmatic policy-makers.CMAJ. 2009 May 12;180(10):1001-3. doi: 10.1503/cmaj.090076. Epub 2009 Apr 16. CMAJ. 2009. PMID: 19372437 Free PMC article. No abstract available.
References
-
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–48. [Reprinted in J Clin Epidemiol 2009;62:499–505.] - PubMed
-
- Sackett DL. Explanatory vs management trials. In: Haynes RB, Sackett DL, Guyatt GH, et al., editors. Clinical epidemiology: how to do clinical practice research. Philadelphia (PA): Lippincott, Williams and Wilkins; 2006.
-
- Gartlehner G, Hansen RA, Nissman D, et al. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59:1040–8. - PubMed
-
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
