Core2 1-6-N-glucosaminyltransferase-I deficiency protects injured arteries from neointima formation in ApoE-deficient mice
- PMID: 19372458
- PMCID: PMC2735567
- DOI: 10.1161/ATVBAHA.109.187716
Core2 1-6-N-glucosaminyltransferase-I deficiency protects injured arteries from neointima formation in ApoE-deficient mice
Abstract
Objective: Core2 1 to 6-N-glucosaminyltransferase-I (C2GlcNAcT-I) plays an important role in optimizing the binding functions of several selectin ligands, including P-selectin glycoprotein ligand. We used apolipoprotein E (ApoE)-deficient atherosclerotic mice to investigate the role of C2GlcNAcT-I in platelet and leukocyte interactions with injured arterial walls, in endothelial regeneration at injured sites, and in the formation of arterial neointima.
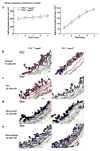
Methods and results: Arterial neointima induced by wire injury was smaller in C2GlcNAcT-I-deficient apoE(-/-) mice than in control apoE(-/-) mice (a 79% reduction in size). Compared to controls, apoE(-/-) mice deficient in C2GlcNAcT-I also demonstrated less leukocyte adhesion on activated platelets in microflow chambers (a 75% reduction), and accumulation of leukocytes at injured areas of mouse carotid arteries was eliminated. Additionally, endothelial regeneration in injured lumenal areas was substantially faster in C2GlcNAcT-I-deficient apoE(-/-) mice than in control apoE(-/-) mice. Endothelial regeneration was associated with reduced accumulation of platelet factor 4 (PF4) at injured sites. PF4 deficiency accelerated endothelial regeneration and protected mice from neointima formation after arterial injury.
Conclusions: C2GlcNAcT-I deficiency suppresses injury-induced arterial neointima formation, and this effect is attributable to decreased leukocyte recruitment to injured vascular walls and increased endothelial regeneration. Both C2GlcNAcT-I and PF4 are promising targets for the treatment of arterial restenosis.
Figures






Similar articles
-
Adenosine receptor A2A deficiency in leukocytes increases arterial neointima formation in apolipoprotein E-deficient mice.Arterioscler Thromb Vasc Biol. 2010 May;30(5):915-22. doi: 10.1161/ATVBAHA.109.202572. Epub 2010 Feb 18. Arterioscler Thromb Vasc Biol. 2010. PMID: 20167656 Free PMC article.
-
Platelet IκB kinase-β deficiency increases mouse arterial neointima formation via delayed glycoprotein Ibα shedding.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):241-8. doi: 10.1161/ATVBAHA.112.300781. Epub 2012 Dec 13. Arterioscler Thromb Vasc Biol. 2013. PMID: 23241410 Free PMC article.
-
Core2 1-6-N-glucosaminyltransferase-I is crucial for the formation of atherosclerotic lesions in apolipoprotein E-deficient mice.Arterioscler Thromb Vasc Biol. 2009 Feb;29(2):180-7. doi: 10.1161/ATVBAHA.108.170969. Epub 2008 Dec 4. Arterioscler Thromb Vasc Biol. 2009. PMID: 19057022 Free PMC article.
-
Platelet CD40 Mediates Leukocyte Recruitment and Neointima Formation after Arterial Denudation Injury in Atherosclerosis-Prone Mice.Am J Pathol. 2018 Jan;188(1):252-263. doi: 10.1016/j.ajpath.2017.09.007. Epub 2017 Oct 14. Am J Pathol. 2018. PMID: 29037856 Free PMC article.
-
Absence of p-selectin, but not intercellular adhesion molecule-1, attenuates neointimal growth after arterial injury in apolipoprotein e-deficient mice.Circulation. 2001 Feb 20;103(7):1000-5. doi: 10.1161/01.cir.103.7.1000. Circulation. 2001. PMID: 11181476
Cited by
-
Blocking CCL5-CXCL4 heteromerization preserves heart function after myocardial infarction by attenuating leukocyte recruitment and NETosis.Sci Rep. 2018 Jul 13;8(1):10647. doi: 10.1038/s41598-018-29026-0. Sci Rep. 2018. PMID: 30006564 Free PMC article.
-
Adenosine receptor A2A deficiency in leukocytes increases arterial neointima formation in apolipoprotein E-deficient mice.Arterioscler Thromb Vasc Biol. 2010 May;30(5):915-22. doi: 10.1161/ATVBAHA.109.202572. Epub 2010 Feb 18. Arterioscler Thromb Vasc Biol. 2010. PMID: 20167656 Free PMC article.
-
Platelet IκB kinase-β deficiency increases mouse arterial neointima formation via delayed glycoprotein Ibα shedding.Arterioscler Thromb Vasc Biol. 2013 Feb;33(2):241-8. doi: 10.1161/ATVBAHA.112.300781. Epub 2012 Dec 13. Arterioscler Thromb Vasc Biol. 2013. PMID: 23241410 Free PMC article.
-
Flank sequences of miR-145/143 and their aberrant expression in vascular disease: mechanism and therapeutic application.J Am Heart Assoc. 2013 Oct 28;2(6):e000407. doi: 10.1161/JAHA.113.000407. J Am Heart Assoc. 2013. PMID: 24166492 Free PMC article.
-
P-selectin glycoprotein ligand-1 plays a crucial role in the selective recruitment of leukocytes into the atherosclerotic arterial wall.Trends Cardiovasc Med. 2009 May;19(4):140-5. doi: 10.1016/j.tcm.2009.07.006. Trends Cardiovasc Med. 2009. PMID: 19818951 Free PMC article. Review.
References
-
- Kuntz RE, Gibson CM, Nobuyoshi M, Baim DS. Generalized model of restenosis after conventional balloon angioplasty, stenting and directional atherectomy. J Am Coll Cardiol. 1993;21(1):15–25. - PubMed
-
- Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res. 1993;73:792–6. - PubMed
-
- Andrews RK, Shen Y, Gardiner EE, Dong JF, Lopez JA, Berndt MC. The glycoprotein Ib-IX-V complex in platelet adhesion and signaling. Thromb Haemost. 1999;82(2):357–64. - PubMed
-
- Phillips DR, Charo IF, Scarborough RM. GPIIb-IIIa - The responsive integrin. Cell. 1991;65:359–62. - PubMed
-
- Manka D, Forlow SB, Sanders JM, Hurwitz D, Bennett DK, Green SA, Ley K, Sarembock IJ. Critical Role of Platelet P-Selectin in the Response to Arterial Injury in Apolipoprotein-E-Deficient Mice. Arterioscler Thromb Vasc Biol. 2004;24(6):1124–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL080133/HL/NHLBI NIH HHS/United States
- R01 HL080569/HL/NHLBI NIH HHS/United States
- R01 HL078679/HL/NHLBI NIH HHS/United States
- HL57345/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P01 HL057345/HL/NHLBI NIH HHS/United States
- HL080569/HL/NHLBI NIH HHS/United States
- HL78679,/HL/NHLBI NIH HHS/United States
- GM62116/GM/NIGMS NIH HHS/United States
- R01 CA033000/CA/NCI NIH HHS/United States
- R01 HL095707/HL/NHLBI NIH HHS/United States
- P01 HL078784/HL/NHLBI NIH HHS/United States
- U54 GM062116/GM/NIGMS NIH HHS/United States
- HL78784/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

