Reduced vessel elasticity alters cardiovascular structure and function in newborn mice
- PMID: 19372465
- PMCID: PMC2800958
- DOI: 10.1161/CIRCRESAHA.108.192054
Reduced vessel elasticity alters cardiovascular structure and function in newborn mice
Abstract
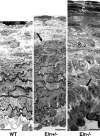
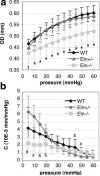
Elastic blood vessels provide capacitance and pulse-wave dampening, which are critically important in a pulsatile circulatory system. By studying newborn mice with reduced (Eln(+/)(-)) or no (Eln(-)(/)(-)) elastin, we determined the effects of altered vessel elasticity on cardiovascular development and function. Eln(-)(/)(-) mice die within 72 hours of birth but are viable throughout fetal development when dramatic cardiovascular structural and hemodynamic changes occur. Thus, newborn Eln(-)(/)(-) mice provide unique insight into how a closed circulatory system develops when the arteries cannot provide the elastic recoil required for normal heart function. Compared with wild type, the Eln(-)(/)(-) aorta has a smaller unloaded diameter and thicker wall because of smooth muscle cell overproliferation and has greatly reduced compliance. Arteries in Eln(-)(/)(-) mice are also tortuous with stenoses and dilations. Left ventricular pressure is 2-fold higher than wild type, and heart function is impaired. Newborn Eln(+/)(-) mice, in contrast, have normal heart function despite left ventricular pressures 25% higher than wild type. The major vessels have smaller unloaded diameters and longer lengths. The Eln(+/)(-) aorta has additional smooth muscle cell layers that appear in the adventitia at or just before birth. These results show that the major adaptive changes in cardiovascular hemodynamics and in vessel wall structure seen in the adult Eln(+/)(-) mouse are defined in late fetal development. Together, these results show that reduced elastin in mice leads to adaptive remodeling, whereas the complete lack of elastin leads to pathological remodeling and death.
Figures




Similar articles
-
The importance of elastin to aortic development in mice.Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H257-64. doi: 10.1152/ajpheart.00194.2010. Epub 2010 May 21. Am J Physiol Heart Circ Physiol. 2010. PMID: 20495146 Free PMC article.
-
Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries.Am J Physiol Heart Circ Physiol. 2005 Sep;289(3):H1209-17. doi: 10.1152/ajpheart.00046.2005. Epub 2005 Apr 29. Am J Physiol Heart Circ Physiol. 2005. PMID: 15863465
-
Elastin haploinsufficiency induces alternative aging processes in the aorta.Rejuvenation Res. 2008 Feb;11(1):97-112. doi: 10.1089/rej.2007.0587. Rejuvenation Res. 2008. PMID: 18173368 Free PMC article.
-
Elastin, arterial mechanics, and stenosis.Am J Physiol Cell Physiol. 2022 May 1;322(5):C875-C886. doi: 10.1152/ajpcell.00448.2021. Epub 2022 Feb 23. Am J Physiol Cell Physiol. 2022. PMID: 35196168 Free PMC article. Review.
-
[Elastin and microfibrils in vascular development and ageing: complementary or opposite roles?].Biol Aujourdhui. 2012;206(2):87-102. doi: 10.1051/jbio/2012009. Epub 2012 Jul 4. Biol Aujourdhui. 2012. PMID: 22748047 Review. French.
Cited by
-
Correlations between transmural mechanical and morphological properties in porcine thoracic descending aorta.J Mech Behav Biomed Mater. 2015 Jul;47:12-20. doi: 10.1016/j.jmbbm.2015.03.004. Epub 2015 Mar 19. J Mech Behav Biomed Mater. 2015. PMID: 25837340 Free PMC article.
-
Hyperelastic remodeling in the intrauterine growth restricted (IUGR) carotid artery in the near-term fetus.J Biomech. 2013 Mar 15;46(5):956-63. doi: 10.1016/j.jbiomech.2012.12.013. Epub 2013 Jan 16. J Biomech. 2013. PMID: 23332229 Free PMC article.
-
Effects of elastin degradation and surrounding matrix support on artery stability.Am J Physiol Heart Circ Physiol. 2012 Feb 15;302(4):H873-84. doi: 10.1152/ajpheart.00463.2011. Epub 2011 Dec 9. Am J Physiol Heart Circ Physiol. 2012. PMID: 22159998 Free PMC article.
-
Function of Ltbp-4L and fibulin-4 in survival and elastogenesis in mice.Dis Model Mech. 2016 Nov 1;9(11):1367-1374. doi: 10.1242/dmm.026005. Epub 2016 Sep 1. Dis Model Mech. 2016. PMID: 27585882 Free PMC article.
-
Twisted blood vessels: symptoms, etiology and biomechanical mechanisms.J Vasc Res. 2012;49(3):185-97. doi: 10.1159/000335123. Epub 2012 Mar 14. J Vasc Res. 2012. PMID: 22433458 Free PMC article. Review.
References
-
- Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. - PubMed
-
- Davis EC. Elastic lamina growth in the developing mouse aorta. J Histochem Cytochem. 1995;43:1115–1123. - PubMed
-
- Huang Y, Guo X, Kassab GS. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am J Physiol Heart Circ Physiol. 2006;290:H657–H664. - PubMed
-
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res. 2003;93:857–865. - PubMed
-
- Keller BB, MacLennan MJ, Tinney JP, Yoshigi M. In vivo assessment of embryonic cardiovascular dimensions and function in day-10.5 to -14.5 mouse embryos. Circ Res. 1996;79:247–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

