Tautomycetin and tautomycin suppress the growth of medullary thyroid cancer cells via inhibition of glycogen synthase kinase-3beta
- PMID: 19372564
- PMCID: PMC2670470
- DOI: 10.1158/1535-7163.MCT-08-0712
Tautomycetin and tautomycin suppress the growth of medullary thyroid cancer cells via inhibition of glycogen synthase kinase-3beta
Erratum in
- Mol Cancer Ther. 2010 May;9(5):1482
Abstract
Medullary thyroid cancer (MTC) is a relatively uncommon neuroendocrine tumor that arises from the calcitonin-secreting parafollicular cells of the thyroid gland. Unfortunately, MTC frequently metastasizes, precluding curative surgical resection and causing significant morbidity. Thus, there is an urgent need for new treatment modalities. Tautomycin and tautomycetin are antifungal antibiotics isolated from Streptomyces spiroverticillatus and Streptomyces griseochromogens, respectively. Glycogen synthase kinase-3beta is a serine/threonine protein kinase that regulates multiple cellular processes and is important in various cancers, including MTC. Treatment with tautomycin and tautomycetin decreased neuroendocrine markers, suppressed hormonal secretion, and inhibited growth through apoptosis in MTC cells. Importantly, we describe a novel action of these compounds: inhibition of glycogen synthase kinase-3beta.
Figures




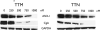
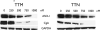
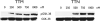


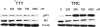
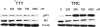
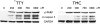
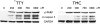
References
-
- Greenblatt DY, Chen H. Palliation of advanced thyroid malignancies. Surg Oncol. 2007;16:237–47. - PubMed
-
- Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist. 2008;13:539–47. - PubMed
-
- Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. - PubMed
-
- Patel DS, Dessalew N, Iqbal P, Bharatam PV. Structure-based approaches in the design of GSK-3 selective inhibitors. Curr Protein Pept Sci. 2007;8:352–64. - PubMed
-
- Garcea G, Manson MM, Neal CP, et al. Glycogen synthase kinase-3 beta; a new target in pancreatic cancer? Curr Cancer Drug Targets. 2007;7:209–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

