Mcl-1 is required for melanoma cell resistance to anoikis
- PMID: 19372583
- PMCID: PMC2689141
- DOI: 10.1158/1541-7786.MCR-08-0358
Mcl-1 is required for melanoma cell resistance to anoikis
Abstract
Melanoma is a particularly aggressive tumor type that exhibits a high level of resistance to apoptosis. The serine/threonine kinase B-RAF is mutated in 50% to 70% of melanomas and protects melanoma cells from anoikis, a form of apoptosis induced by lack of adhesion or adhesion to an inappropriate matrix. Mutant B-RAF down-regulates two BH3-only proapoptotic proteins, Bim(EL) and Bad. BH3-only proteins act, at least in part, by sequestering prosurvival Bcl-2 family proteins and preventing them from inhibiting the mitochondrial apoptotic pathway. Several Bcl-2 proteins are up-regulated in melanoma; however, the mechanisms of up-regulation and their role in melanoma resistance to anoikis remain unclear. Using RNA interference, we show that depletion of Mcl-1 renders mutant B-RAF melanoma cells sensitive to anoikis. By contrast, minor effects were observed following depletion of either Bcl-2 or Bcl-(XL). Mcl-1 expression is enhanced in melanoma cell lines compared with melanocytes and up-regulated by the B-RAF-MEK-extracellular signal-regulated kinase 1/2 pathway through control of Mcl-1 protein turnover. Similar to B-RAF knockdown cells, adhesion to fibronectin protected Mcl-1 knockdown cells from apoptosis. Finally, expression of Bad, which does not sequester Mcl-1, further augmented apoptosis in nonadherent Mcl-1 knockdown cells. Together, these data support the notion that BH3 mimetic compounds that target Mcl-1 may be effective for the treatment of melanoma in combinatorial strategies with agents that disrupt fibronectin-integrin signaling.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


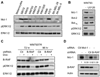
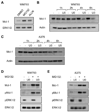


References
-
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. - PubMed
-
- Letai A, Bassik MC, Walensky LD, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. - PubMed
-
- Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. - PubMed
-
- Willis SN, Fletcher JI, Kaufmann T, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

