Identification and characterization of the Na+/H+ antiporter Nhas3 from the thylakoid membrane of Synechocystis sp. PCC 6803
- PMID: 19372598
- PMCID: PMC2713527
- DOI: 10.1074/jbc.M109.001875
Identification and characterization of the Na+/H+ antiporter Nhas3 from the thylakoid membrane of Synechocystis sp. PCC 6803
Abstract
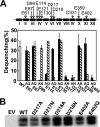
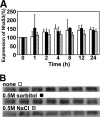
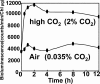
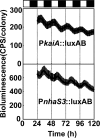
Na+/H+ antiporters influence proton or sodium motive force across the membrane. Synechocystis sp. PCC 6803 has six genes encoding Na+/H+ antiporters, nhaS1-5 and sll0556. In this study, the function of NhaS3 was examined. NhaS3 was essential for growth of Synechocystis, and loss of nhaS3 was not complemented by expression of the Escherichia coli Na+/H+ antiporter NhaA. Membrane fractionation followed by immunoblotting as well as immunogold labeling revealed that NhaS3 was localized in the thylakoid membrane of Synechocystis. NhaS3 was shown to be functional over a pH range from pH 6.5 to 9.0 when expressed in E. coli. A reduction in the copy number of nhaS3 in the Synechocystis genome rendered the cells more sensitive to high Na+ concentrations. NhaS3 had no K+/H+ exchange activity itself but enhanced K+ uptake from the medium when expressed in an E. coli potassium uptake mutant. Expression of nhaS3 increased after shifting from low CO2 to high CO2 conditions. Expression of nhaS3 was also found to be controlled by the circadian rhythm. Gene expression peaked at the beginning of subjective night. This coincided with the time of the lowest rate of CO2 consumption caused by the ceasing of O2-evolving photosynthesis. This is the first report of a Na+/H+ antiporter localized in thylakoid membrane. Our results suggested a role of NhaS3 in the maintenance of ion homeostasis of H+, Na+, and K+ in supporting the conversion of photosynthetic products and in the supply of energy in the dark.
Figures




Similar articles
-
Na + -driven pH regulation by Na+/H+ antiporters promotes photosynthetic efficiency in cyanobacteria.Plant Physiol. 2024 Dec 23;197(1):kiae562. doi: 10.1093/plphys/kiae562. Plant Physiol. 2024. PMID: 39446395 Free PMC article.
-
Functional expression in Escherichia coli of low-affinity and high-affinity Na(+)(Li(+))/H(+) antiporters of Synechocystis.J Bacteriol. 2001 Feb;183(4):1376-84. doi: 10.1128/JB.183.4.1376-1384.2001. J Bacteriol. 2001. PMID: 11157951 Free PMC article.
-
Functional analysis of the Na+/H+ antiporter encoding genes of the cyanobacterium Synechocystis PCC 6803.Biochemistry (Mosc). 2002 Apr;67(4):432-40. doi: 10.1023/a:1015281906254. Biochemistry (Mosc). 2002. PMID: 11996656
-
Na(+)/H(+) antiporters.Biochim Biophys Acta. 2001 May 1;1505(1):144-57. doi: 10.1016/s0005-2728(00)00284-x. Biochim Biophys Acta. 2001. PMID: 11248196 Review.
-
pH homeostasis and ATP synthesis: studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species.Extremophiles. 1998 Aug;2(3):217-22. doi: 10.1007/s007920050063. Extremophiles. 1998. PMID: 9783168 Review.
Cited by
-
Comparative analysis of kdp and ktr mutants reveals distinct roles of the potassium transporters in the model cyanobacterium Synechocystis sp. strain PCC 6803.J Bacteriol. 2015 Feb 15;197(4):676-87. doi: 10.1128/JB.02276-14. Epub 2014 Oct 13. J Bacteriol. 2015. PMID: 25313394 Free PMC article.
-
Na + -driven pH regulation by Na+/H+ antiporters promotes photosynthetic efficiency in cyanobacteria.Plant Physiol. 2024 Dec 23;197(1):kiae562. doi: 10.1093/plphys/kiae562. Plant Physiol. 2024. PMID: 39446395 Free PMC article.
-
Dissecting structure and function of the monovalent cation/H+ antiporters Mdm38 and Ylh47 in Saccharomyces cerevisiae.J Bacteriol. 2024 Aug 22;206(8):e0018224. doi: 10.1128/jb.00182-24. Epub 2024 Jul 31. J Bacteriol. 2024. PMID: 39082862 Free PMC article.
-
The Global Influence of Sodium on Cyanobacteria in Resuscitation from Nitrogen Starvation.Biology (Basel). 2023 Jan 19;12(2):159. doi: 10.3390/biology12020159. Biology (Basel). 2023. PMID: 36829438 Free PMC article.
-
Salt acclimation of cyanobacteria and their application in biotechnology.Life (Basel). 2014 Dec 29;5(1):25-49. doi: 10.3390/life5010025. Life (Basel). 2014. PMID: 25551682 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

