Aldosterone stimulates elastogenesis in cardiac fibroblasts via mineralocorticoid receptor-independent action involving the consecutive activation of Galpha13, c-Src, the insulin-like growth factor-I receptor, and phosphatidylinositol 3-kinase/Akt
- PMID: 19372600
- PMCID: PMC2713569
- DOI: 10.1074/jbc.M109.008748
Aldosterone stimulates elastogenesis in cardiac fibroblasts via mineralocorticoid receptor-independent action involving the consecutive activation of Galpha13, c-Src, the insulin-like growth factor-I receptor, and phosphatidylinositol 3-kinase/Akt
Abstract
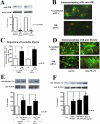
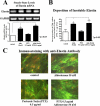
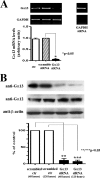
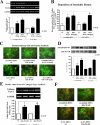
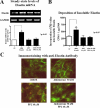
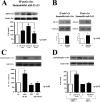
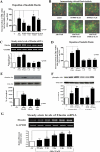
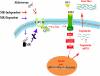
We previously demonstrated that aldosterone, which stimulates collagen production through the mineralocorticoid receptor (MR)-dependent pathway, also induces elastogenesis via a parallel MR-independent mechanism involving insulin-like growth factor-I receptor (IGF-IR) signaling. The present study provides a more detailed explanation of this signaling pathway. Our data demonstrate that small interfering RNA-driven elimination of MR in cardiac fibroblasts does not inhibit aldosterone-induced IGF-IR phosphorylation and subsequent increase in elastin production. These results exclude the involvement of the MR in aldosterone-induced increases in elastin production. Results of further experiments aimed at identifying the upstream signaling component(s) that might be activated by aldosterone also eliminate the putative involvement of pertussis toxin-sensitive Galphai proteins, which have previously been shown to be responsible for some MR-independent effects of aldosterone. Instead, we found that small interfering RNA-dependent elimination of another heterotrimeric G protein, Galpha13, eliminates aldosterone-induced elastogenesis. We further demonstrate that aldosterone first engages Galpha13 and then promotes its transient interaction with c-Src, which constitutes a prerequisite step for aldosterone-dependent activation of the IGF-IR and propagation of consecutive downstream elastogenic signaling involving phosphatidylinositol 3-kinase/Akt. In summary, the data we present reveal new details of an MR-independent cellular signaling pathway through which aldosterone stimulates elastogenesis in human cardiac fibroblasts.
Figures
References
-
- Jennings D. L., Kalus J. S., O'Dell K. M. ( 2005) Pharmacotherapy 25, 1126– 1133 - PubMed
-
- Connell J. M., Davies E. ( 2005) J. Endocrinol. 186, 1– 20 - PubMed
-
- Weber K. T., Brilla C. G. ( 1991) Circulation 83, 1849– 1865 - PubMed
-
- Brilla C. G., Matsubara L. S., Weber K. T. ( 1993) Am. J. Cardiol. 71, 12A– 16A - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

