Dynamic properties of pH-dependent structural organization of the amyloidogenic beta-protein (1-40)
- PMID: 19372746
- PMCID: PMC2676741
- DOI: 10.4161/pri.3.1.8388
Dynamic properties of pH-dependent structural organization of the amyloidogenic beta-protein (1-40)
Abstract
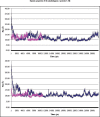
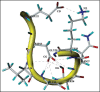
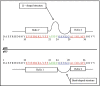
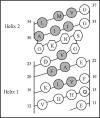
The structural organization of the amyloidogenic beta-protein containing 40 amino acid residues (Abeta40) was studied by the high temperature molecular dynamics simulations in the acidic (pH approximately 3) and basic (pH approximately 8) pH regions. The obtained data suggest that the central Ala21-Gly29 segment of Abeta40 can adopt folded and partially unfolded structures. At the basic pH, this segment forms folded structures stabilized by electrostatic interactions and hydrogen bonds. At the acidic pH, it forms partially unfolded structures. Two other segments flanking to the central segment exhibit the propensity to adopt unstable interconverting alpha-helical, 3(10)-helical and turn-like structures. One of these segments is comprised of the Ala30-Val36 residues at both of the considered pHs. The second segment is comprised of the Glu11-Phe20 at the basic pH and of the Glu11-Val24 residues at the acidic pHs. The revealed pH-dependent structuration of the Abeta40 allowed us to suggest a possible scenario for initial Abeta aggregation. According to this scenario, the occurrence of the partially unfolded states of the Ala21-Gly29 segment plays main role in the Abeta oligomerization process.
Figures


Similar articles
-
Effects of the Arctic (E22-->G) mutation on amyloid beta-protein folding: discrete molecular dynamics study.J Am Chem Soc. 2008 Dec 24;130(51):17413-22. doi: 10.1021/ja804984h. J Am Chem Soc. 2008. PMID: 19053400
-
Identification of key regions and residues controlling Aβ folding and assembly.Sci Rep. 2017 Oct 3;7(1):12434. doi: 10.1038/s41598-017-10845-6. Sci Rep. 2017. PMID: 28974765 Free PMC article.
-
Distinct early folding and aggregation properties of Alzheimer amyloid-beta peptides Abeta40 and Abeta42: stable trimer or tetramer formation by Abeta42.J Biol Chem. 2006 Aug 25;281(34):24414-22. doi: 10.1074/jbc.M602363200. Epub 2006 Jun 29. J Biol Chem. 2006. PMID: 16809342
-
Effect of pH on Aβ42 Monomer and Fibril-like Oligomers-Decoding in Silico of the Roles of pK Values of Charged Residues.Chemphyschem. 2018 May 7;19(9):1103-1116. doi: 10.1002/cphc.201701384. Epub 2018 Mar 5. Chemphyschem. 2018. PMID: 29380494
-
Molecular dynamics simulations of human prion protein: importance of correct treatment of electrostatic interactions.Biochemistry. 1999 Oct 19;38(42):13862-76. doi: 10.1021/bi991469d. Biochemistry. 1999. PMID: 10529232
Cited by
-
α-Synuclein misfolding assessed with single molecule AFM force spectroscopy: effect of pathogenic mutations.Biochemistry. 2013 Oct 22;52(42):7377-86. doi: 10.1021/bi401037z. Epub 2013 Oct 10. Biochemistry. 2013. PMID: 24066883 Free PMC article.
-
The Origin of Discrepancies between Predictions and Annotations in Intrinsically Disordered Proteins.Biomolecules. 2023 Sep 25;13(10):1442. doi: 10.3390/biom13101442. Biomolecules. 2023. PMID: 37892124 Free PMC article.
-
Nanoimaging for prion related diseases.Prion. 2010 Oct-Dec;4(4):265-74. doi: 10.4161/pri.4.4.13125. Epub 2010 Oct 23. Prion. 2010. PMID: 20724837 Free PMC article. Review.
-
Potential sources of interference on Abeta immunoassays in biological samples.Alzheimers Res Ther. 2012 Oct 17;4(5):39. doi: 10.1186/alzrt142. eCollection 2012. Alzheimers Res Ther. 2012. PMID: 23082750 Free PMC article. Review.
-
Dimer formation enhances structural differences between amyloid β-protein (1-40) and (1-42): an explicit-solvent molecular dynamics study.PLoS One. 2012;7(4):e34345. doi: 10.1371/journal.pone.0034345. Epub 2012 Apr 11. PLoS One. 2012. PMID: 22509291 Free PMC article.
References
-
- Hardy J, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer's disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. - PubMed
-
- Petkova AT, Leapman RD, Guo Z, Yau W-M, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Altzheimer's β-amyloid fibrils. Science. 2005;307:262–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
