A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells
- PMID: 19373231
- PMCID: PMC2688697
- DOI: 10.1038/nprot.2009.33
A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells
Abstract
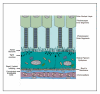
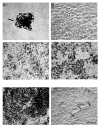
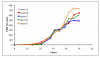
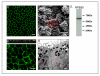
We provide our detailed, standardized in vitro protocol for the culture and differentiation of human retinal pigment epithelial (RPE) cells into a highly polarized and functional monolayer. Disruption of the polarized RPE function plays an important role in the pathogenesis of common blinding disorders of the retina. The availability of this polarized RPE monolayer allows for reproducible evaluation of RPE function, modeling of RPE dysfunction in retinal disease and in vitro evaluation of new therapies. The protocol, which takes approximately 6 weeks to complete, describes the culture of RPE from human fetal donor eyes, the differentiation of these cells into a polarized monolayer with high transepithelial resistance and morphologic characteristics that mimic the RPE monolayer in vivo. By modifying the procedure for initial isolation of pure RPE cells and the culture conditions used in existing protocols, we have established a standardized protocol that provides highly reproducible RPE monolayers from the same donor eye.
Figures









References
-
- Thumann G, Hoffmann S, Hinton DR. Cell biology of the retinal pigment epithelium. In: Ryan SJ, editor. Retina. 4rd ed Vol. 1. Elsevier Mosby; Philadelphia, USA: 2006. pp. 137–152.
-
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. - PubMed
-
- Marmor MF, Abdul-Rahim AS, Cohen DS. The effect of metabolic inhibitors on retinal adhesion and subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1980;19:893–903. - PubMed
-
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

