CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes
- PMID: 19373246
- PMCID: PMC5848464
- DOI: 10.1038/cdd.2009.43
CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes
Abstract
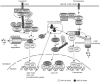
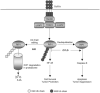
Protein ubiquitination is a reversible reaction, in which the ubiquitin chains are deconjugated by a family of deubiquitinases (DUBs). The presence of a large number of DUBs suggests that they likely possess certain levels of substrate selectivity and functional specificity. Indeed, recent studies show that a tumor suppressor DUB, cylindromatosis (CYLD), has a predominant role in the regulation of NF-kappaB, a transcription factor that promotes cell survival and oncogenesis. NF-kappaB activation involves attachment of K63-linked ubiquitin chains to its upstream signaling factors, which is thought to facilitate protein-protein interactions in the assembly of signaling complexes. By deconjugating these K63-linked ubiquitin chains, CYLD negatively regulates NF-kappaB activation, which may contribute to its tumor suppressor function. CYLD also regulates diverse physiological processes, ranging from immune response and inflammation to cell cycle progression, spermatogenesis, and osteoclastogenesis. Interestingly, CYLD itself is subject to different mechanisms of regulation.
Figures
Similar articles
-
Deubiquitinases in the regulation of NF-κB signaling.Cell Res. 2011 Jan;21(1):22-39. doi: 10.1038/cr.2010.166. Epub 2010 Nov 30. Cell Res. 2011. PMID: 21119682 Free PMC article. Review.
-
Suppression of LUBAC-mediated linear ubiquitination by a specific interaction between LUBAC and the deubiquitinases CYLD and OTULIN.Genes Cells. 2014 Mar;19(3):254-72. doi: 10.1111/gtc.12128. Epub 2014 Jan 26. Genes Cells. 2014. PMID: 24461064
-
Subquinocin, a small molecule inhibitor of CYLD and USP-family deubiquitinating enzymes, promotes NF-κB signaling.Biochem Biophys Res Commun. 2020 Mar 26;524(1):1-7. doi: 10.1016/j.bbrc.2019.12.049. Epub 2019 Dec 30. Biochem Biophys Res Commun. 2020. PMID: 31898971
-
Proinflammatory mediators alter expression of nuclear factor kappa B-regulating deubiquitinases in sinonasal epithelial cells.Int Forum Allergy Rhinol. 2015 Jul;5(7):583-9. doi: 10.1002/alr.21538. Epub 2015 Apr 24. Int Forum Allergy Rhinol. 2015. PMID: 25907801 Free PMC article.
-
Regulation of NF-κB by deubiquitinases.Immunol Rev. 2012 Mar;246(1):107-24. doi: 10.1111/j.1600-065X.2012.01100.x. Immunol Rev. 2012. PMID: 22435550 Free PMC article. Review.
Cited by
-
CYLD regulates RhoA activity by modulating LARG ubiquitination.PLoS One. 2013;8(2):e55833. doi: 10.1371/journal.pone.0055833. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405219 Free PMC article.
-
Cycles of ubiquitination and deubiquitination critically regulate growth factor-mediated activation of Akt signaling.Sci Signal. 2013 Jan 8;6(257):ra3. doi: 10.1126/scisignal.2003197. Sci Signal. 2013. PMID: 23300340 Free PMC article.
-
CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt.Nat Commun. 2012 Apr 10;3:771. doi: 10.1038/ncomms1776. Nat Commun. 2012. PMID: 22491319 Free PMC article.
-
Interactions between SARS coronavirus 2 papain-like protease and immune system: A potential drug target for the treatment of COVID-19.Scand J Immunol. 2021 Oct;94(4):e13044. doi: 10.1111/sji.13044. Epub 2021 Aug 10. Scand J Immunol. 2021. PMID: 33872387 Free PMC article. Review.
-
UBE2N is essential for maintenance of skin homeostasis and suppression of inflammation.bioRxiv [Preprint]. 2023 Dec 4:2023.12.01.569631. doi: 10.1101/2023.12.01.569631. bioRxiv. 2023. Update in: J Invest Dermatol. 2024 Dec;144(12):2742-2753. doi: 10.1016/j.jid.2024.04.017. PMID: 38105982 Free PMC article. Updated. Preprint.
References
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. - PubMed
-
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. - PubMed
-
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

