The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis
- PMID: 19373441
- PMCID: PMC2925118
- DOI: 10.1007/s12029-009-9058-y
The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis
Abstract
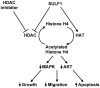
Introduction: Human sulfatase 1 (SULF1) was recently identified and shown to desulfate cellular heparan sulfate proteoglycans (HSPGs). Since sulfated HSPGs serve as co-receptors for many growth factors and cytokines, SULF1 was predicted to modulate growth factor and cytokine signaling.
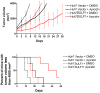
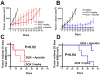
Discussion: The role of SULF1 in growth factor signaling and its effects on human tumorigenesis are under active investigation. Initial results show that SULF1 inhibits the co-receptor function of HSPGs in multiple receptor tyrosine kinase signaling pathways, particularly by the heparin binding growth factors--fibroblast growth factor 2, vascular endothelial growth factor, hepatocyte growth factor, PDGF, and heparin-binding epidermal growth factor (HB-EGF). SULF1 is downregulated in the majority of cancer cell lines examined, and forced expression of SULF1 decreases cell proliferation, migration, and invasion. SULF1 also promotes drug-induced apoptosis of cancer cells in vitro and inhibits tumorigenesis and angiogenesis in vivo.
Conclusion: Strategies targeting SULF1 or the interaction between SULF1 and the related sulfatase 2 will potentially be important in developing novel cancer therapies.
Figures


References
-
- Diez-Roux G, Ballabio A. Sulfatases and human disease. Annu Rev Genomics Hum Genet. 2005;6:355–379. - PubMed
-
- Shridhar V, Sen A, Chien J, Staub J, Avula R, Kovats S, Lee J, Lillie J, Smith DI. Identification of underexpressed genes in early- and late-stage primary ovarian tumors by suppression subtraction hybridization. Cancer research. 2002;62(1):262–270. - PubMed
-
- Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr Substrate specificity and domain functions of extracellular heparan sulfate 6-O-endosulfatases, QSulf1 and QSulf2. The Journal of biological chemistry. 2006;281(8):4969–4976. - PubMed
-
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293(5535):1663–1666. - PubMed
-
- Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR, Shridhar V. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. The Journal of biological chemistry. 2003;278(25):23107–23117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
