Sodium selenite increases the activity of the tumor suppressor protein, PTEN, in DU-145 prostate cancer cells
- PMID: 19373605
- PMCID: PMC4049328
- DOI: 10.1080/01635580802521338
Sodium selenite increases the activity of the tumor suppressor protein, PTEN, in DU-145 prostate cancer cells
Abstract
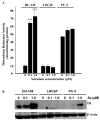
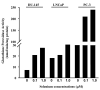
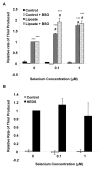
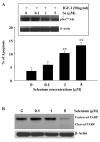
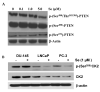
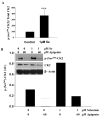
Epidemiological and clinical data suggest that selenium may prevent prostate cancer; however, the cellular effects of selenium in malignant prostate cells are not well understood. We previously reported that the activity of the tumor suppressor PTEN is modulated by thioredoxin (Trx) in a RedOx-dependent manner. In this study, we demonstrated that the activity of Trx reductase (TR) is increased by sevenfold in the human prostate cancer cell line, DU-145, after 5 days of sodium selenite (Se) treatment. The treatment of DU-145 cells with increasing concentrations of Se induced an increase in PTEN lipid phosphatase activity by twofold, which correlated with a decrease in phospho-ser(473)-Akt, and an increase in phospho-Ser(370)-PTEN levels. Se also increased casein kinase-2 (CK2) activity; and the use of apigenin, an inhibitor of CK2, revealed that the regulation of the tumor suppressor PTEN by Se may be achieved via both the Trx-TR system and the RedOx control of the kinase involved in the regulation of PTEN activity.
Figures


Similar articles
-
PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells.Cell Death Dis. 2013 Feb 7;4(2):e481. doi: 10.1038/cddis.2013.3. Cell Death Dis. 2013. PMID: 23392169 Free PMC article.
-
Mechanisms of the regulation of thioredoxin reductase activity in cancer cells by the chemopreventive agent selenium.Cancer Res. 1997 Nov 1;57(21):4965-70. Cancer Res. 1997. PMID: 9354464
-
Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity.Arch Biochem Biophys. 2004 Sep 15;429(2):123-33. doi: 10.1016/j.abb.2004.04.020. Arch Biochem Biophys. 2004. PMID: 15313215
-
Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells.Proc Natl Acad Sci U S A. 2000 Mar 28;97(7):3207-12. doi: 10.1073/pnas.97.7.3207. Proc Natl Acad Sci U S A. 2000. PMID: 10716737 Free PMC article.
-
Selenium and the thioredoxin and glutaredoxin systems.Biomed Environ Sci. 1997 Sep;10(2-3):271-9. Biomed Environ Sci. 1997. PMID: 9315320 Review.
Cited by
-
Novel library of selenocompounds as kinase modulators.Molecules. 2011 Jul 27;16(8):6349-64. doi: 10.3390/molecules16086349. Molecules. 2011. PMID: 21796074 Free PMC article.
-
Influence of concurrent medications on outcomes of men with prostate cancer included in the TAX 327 study.Can Urol Assoc J. 2013 Jan-Feb;7(1-2):E74-81. doi: 10.5489/cuaj.267. Can Urol Assoc J. 2013. PMID: 23671512 Free PMC article.
-
PTEN modulators: a patent review.Expert Opin Ther Pat. 2013 May;23(5):569-80. doi: 10.1517/13543776.2013.768985. Epub 2013 Feb 5. Expert Opin Ther Pat. 2013. PMID: 23379765 Free PMC article. Review.
-
Treatment Stratification of Patients with Metastatic Castration-Resistant Prostate Cancer by Machine Learning.iScience. 2020 Feb 21;23(2):100804. doi: 10.1016/j.isci.2019.100804. Epub 2019 Dec 26. iScience. 2020. PMID: 31978751 Free PMC article.
-
PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells.Cell Death Dis. 2013 Feb 7;4(2):e481. doi: 10.1038/cddis.2013.3. Cell Death Dis. 2013. PMID: 23392169 Free PMC article.
References
-
- Linder MC. Nutrition and metabolism trace elements. In: Linder MC, editor. Nutritional biochemistry and metabolism with clinical applications. Elsevier; New York: 1990.
-
- Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1:1059–1066. - PubMed
-
- Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–12. - PubMed
-
- Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–252. - PubMed
-
- Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
