Modulation of HSP27 alters hypoxia-induced endothelial permeability and related signaling pathways
- PMID: 19373869
- PMCID: PMC2891512
- DOI: 10.1002/jcp.21773
Modulation of HSP27 alters hypoxia-induced endothelial permeability and related signaling pathways
Abstract
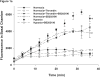
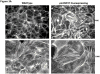
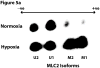
This manuscript describes how the permeability of pulmonary artery microvascular endothelial cell (RPMEC) monolayer is elevated by hypoxia and the role played by HSP27 phosphorylation. p38 MAP kinase activation leading to HSP27 phosphorylation was previously shown by our laboratory to alter the actin cytoskeleton and tethering properties of RPMEC. This effect was independent of hypoxia-induced contractility which was ROCK-dependent rather than HSP27-dependent. Results described here show that increased HSP27 phosphorylation not only does not underlie hypoxia-induced permeability, but may actually augment the endothelial barrier. Hypoxia causes gap formation between RPMEC and increases MLC2 phosphorylation. The phosphorylation of MYPT1, which inhibits MLC2 phosphatase, is also increased in hypoxia. In addition, FAK phosphorylation, which alters focal adhesion signaling, is increased in hypoxia. Overexpressing phosphomimicking HSP27 (pmHSP27), which induces significant actin stress fiber formation, surprisingly renders RPMEC resistant to hypoxia- or TGFbeta-induced permeability. siRNA against pmHSP27 reverses the increased actin stress fiber formation in pmHSP27-overexpressing cells, and disrupting actin stress fibers in pmHSP27-overexpressing RPMEC renders them more susceptible to hypoxia. Finally, hypoxia-induced gap formation, as well as phosphorylation of MLC2, MYPT1 and FAK are almost abolished by overexpressing pmHSP27 in RPMEC. These effects of pmHSP27 overexpression might represent decreased cytoskeletal plasticity and increased tethering which counteracts permeability-inducing contractility. Thus hypoxia activates two pathways one leading to contractility and increased permeability, the other leading to actin stress fibers, stronger adhesion, and reduced permeability. Altering HSP27 phosphorylation, which tips the balance towards decreased permeability, might be targeted in managing endothelial barrier dysfunction.
Figures












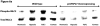
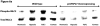
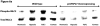
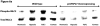


References
-
- Akiyama K, Akopian G, Jinadasa P, Gluckman TL, Terhakopian A, Massey B, Bing RJ. Myocardial infarction and regulatory myosin light chain. J Mol Cell Cardiol. 1997;29(10):2641–2652. - PubMed
-
- Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol. 1999;277(5 Pt 1):L1057–1065. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. - PubMed
-
- An SS, Pennella CM, Gonnabathula A, Chen J, Wang N, Gaestel M, Hassoun PM, Fredberg JJ, Kayyali US. Hypoxia alters biophysical properties of endothelial cells via p38 MAPK- and Rho kinase-dependent pathways. Am J Physiol Cell Physiol. 2005;289(3):C521–530. - PubMed
-
- Baudry N, Danialou G, Boczkowski J, Vicaut E. In vivo study of the effect of systemic hypoxia on leukocyte-endothelium interactions. Am J Respir Crit Care Med. 1998;158(2):477–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

