Induction of DNA damage response by the supravital probes of nucleic acids
- PMID: 19373929
- PMCID: PMC2749728
- DOI: 10.1002/cyto.a.20727
Induction of DNA damage response by the supravital probes of nucleic acids
Abstract
The aim of this study was to assess the potential DNA damage response (DDR) to four supravitally used biomarkers Hoechst 33342 (Ho 42), DRAQ5, DyeCycle Violet (DCV), and SYTO 17. A549 cells were exposed to these biomarkers at concentrations generally applied to live cells and their effect on histone H2AX (Ser 139), p53 (Ser15), ATM (Ser1981), and Chk2 (Thr68) phosphorylation was assessed using phospho-specific Abs. Short-term treatment with Ho 42 led to modest degree of ATM activation with no evidence of H2AX, Chk2, or p53 phosphorylation. However, pronounced ATM, Chk2, and p53 phosphorylation and perturbed G(2) progression were seen after 18 h. While short-term treatment with DRAQ5 induced ATM activation with no effect on H2AX, Chk2, and p53, dramatic changes marked by a high degree of H2AX, ATM, Chk2, and p53 phosphorylation, all occurring predominantly in S phase cells, and a block in cell cycle progression, were seen after 18 h exposure. These changes suggest that the DRAQ5-induced DNA lesions may become converted into double-strand DNA breaks during replication. Exposure to DCV also led to an increase in the level of activated ATM and Chk2 as well as of phosphorylated p53 and accumulation of cells in G(2)M and S phase. Exposure to SYTO 17 had no significant effect on any of the measured parameters. The data indicate that supravital use of Ho 42, DRAQ5, and DCV induces various degrees of DDR, including activation of ATM, Chk2 and p53, which may have significant consequences on regulatory cell cycle pathways and apoptosis.
Figures
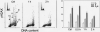
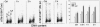
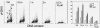
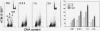





References
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. - PubMed
-
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. - PubMed
-
- Lavin MF, Kozlov S. ATM activation and DNA damage response. Cell Cycle. 2007;6:931–942. - PubMed
-
- Bartkova J, Bakkenist CJ, Rajpert-De Meyts E, Skakkebaek NE, Sehested M, Lukas J, Kastan MB, Bartek J. ATM activation in normal human tissues and testicular cancer. Cell Cycle. 2005;4:838–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

