Coordination chemistry of [HFe(CN)(2)(CO)(3)](-) and its derivatives: toward a model for the iron subsite of the [NiFe]-hydrogenases
- PMID: 19374433
- PMCID: PMC2732431
- DOI: 10.1021/ic900200s
Coordination chemistry of [HFe(CN)(2)(CO)(3)](-) and its derivatives: toward a model for the iron subsite of the [NiFe]-hydrogenases
Abstract
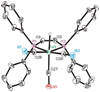
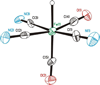
The photoreaction of Fe(CO)(5) and cyanide salts in MeCN solution affords the dianion [Fe(CN)(2)(CO)(3)](2-), conveniently isolated as [K(18-crown-6)](2)[Fe(CN)(2)(CO)(3)]. Solutions of [Fe(CN)(2)(CO)(3)](2-) oxidize irreversibly at -600 mV (vs Ag/AgCl) to give primarily [Fe(CN)(3)(CO)(3)](-). Protonation of the dianion affords the hydride [K(18-crown-6)][HFe(CN)(2)(CO)(3)] with a pK(a) approximately 17 (MeCN). The ferrous hydride exhibits enhanced electrophilicity vs its dianionic precursor, which resists substitution. Treatment of [K(18-crown-6)][Fe(CN)(2)(CO)(3)] with tertiary phosphines and phosphites gives isomeric mixtures of [HFe(CN)(2)(CO)(2)L](-) (L = P(OPh)(3) and PPh(3)). Carbonyl substitution on [1H(CO)(2)](-) by P(OPh)(3) is first-order in both the phosphite and iron (k = 0.18 M(-1) s(-1) at 22 degrees C) with DeltaH(double dagger) = 51.6 kJ mol(-1) and DeltaS(double dagger) = -83.0 J K(-1) mol(-1). These ligands are displaced under an atmosphere of CO. With cis-Ph(2)PCH=CHPPh(2) (dppv), we obtained the monocarbonyl, [HFe(CN)(2)(CO)(dppv)](-), a highly basic hydride (pK(a) > 23.3) that rearranges in solution to a single isomer. Treatment of [K(18-crown-6)][HFe(CN)(2)(CO)(3)] with Et(4)NCN resulted in rapid deprotonation to give [Fe(CN)(2)(CO)(3)](2-) and HCN. The tricyano hydride [HFe(CN)(3)(CO)(2)](2-) is prepared by the reaction of [HFe(CN)(2)(CO)(2)(PPh(3))](-) and [K(18-crown-6)]CN. Similar to the phosphine and phosphite derivatives, [HFe(CN)(3)(CO)(2)](2-) exists as a mixture of all three possible isomers. Protonation of the hydrides [HFe(CN)(2)(CO)(dppv)](-) and [HFe(CN)(3)(CO)(2)](-) in acetonitrile solutions releases H(2) and gives the corresponding acetonitrile complexes [K(18-crown-6)][Fe(CN)(3)(NCMe)(CO)(2)] and Fe(CN)(2)(NCMe)(CO)(dppv). Alkylation of [K(18-crown-6)](2)[Fe(CN)(2)(CO)(3)] with MeOTf gives the thermally unstable [MeFe(CN)(2)(CO)(3)](-), which was characterized spectroscopically at -40 degrees C. Reaction of dppv with [MeFe(CN)(2)(CO)(3)](-) gives the acetyl complex, [Fe(CN)(2)(COMe)(CO)(dppv)](-). Whereas [Fe(CN)(2)(CO)(3)](2-) undergoes protonation and methylation at Fe, acid chlorides give the iron(0) N-acylisocyanides [Fe(CN)(CO)(3)(CNCOR)](-) (R = Ph, CH(3)). The solid state structures of [K(18-crown-6)][HFe(CN)(2)(CO)(dppv)], Fe(CN)(2)(NCMe)(CO)(dppv), and [K(18-crown-6)](2)[HFe(CN)(3)(CO)(2)] were confirmed crystallographically. In all three cases, the cyanide ligands are cis to the hydride or acetonitrile ligands.
Figures


Similar articles
-
Isomerization of the hydride complexes [HFe2(SR)2(PR3)(x)(CO)(6-x)]+ (x = 2, 3, 4) relevant to the active site models for the [FeFe]-hydrogenases.Dalton Trans. 2010 Mar 28;39(12):3011-9. doi: 10.1039/b910147k. Epub 2009 Sep 16. Dalton Trans. 2010. PMID: 20221534 Free PMC article.
-
Chelate control of diiron(I) dithiolates relevant to the [Fe-Fe]- hydrogenase active site.Inorg Chem. 2007 Mar 5;46(5):1655-64. doi: 10.1021/ic0618706. Epub 2007 Feb 6. Inorg Chem. 2007. PMID: 17279743 Free PMC article.
-
Diiron azadithiolates as models for the [FeFe]-hydrogenase active site and paradigm for the role of the second coordination sphere.Acc Chem Res. 2015 Jul 21;48(7):2107-16. doi: 10.1021/acs.accounts.5b00177. Epub 2015 Jun 16. Acc Chem Res. 2015. PMID: 26079848 Free PMC article.
-
Mixed-valence nickel-iron dithiolate models of the [NiFe]-hydrogenase active site.Inorg Chem. 2012 Feb 20;51(4):2338-48. doi: 10.1021/ic202329y. Epub 2012 Feb 3. Inorg Chem. 2012. PMID: 22304696 Free PMC article.
-
New nitrosyl derivatives of diiron dithiolates related to the active site of the [FeFe]-hydrogenases.Inorg Chem. 2008 Dec 15;47(24):11816-24. doi: 10.1021/ic801542w. Inorg Chem. 2008. PMID: 19007207 Free PMC article.
Cited by
-
Hydride-containing models for the active site of the nickel-iron hydrogenases.J Am Chem Soc. 2010 Oct 27;132(42):14877-85. doi: 10.1021/ja105312p. J Am Chem Soc. 2010. PMID: 20925337 Free PMC article.
-
Synthesis and Dynamics of Ferrous Polychalcogenides [Fe(Ex)(CN)2(CO)2]2- (E = S, Se, or Te).Inorg Chem. 2022 May 30;61(21):8241-8249. doi: 10.1021/acs.inorgchem.2c00684. Epub 2022 May 13. Inorg Chem. 2022. PMID: 35561009 Free PMC article.
-
Hydrogenase Enzymes and Their Synthetic Models: The Role of Metal Hydrides.Chem Rev. 2016 Aug 10;116(15):8693-749. doi: 10.1021/acs.chemrev.6b00180. Epub 2016 Jun 29. Chem Rev. 2016. PMID: 27353631 Free PMC article. Review.
-
The iron-site structure of [Fe]-hydrogenase and model systems: an X-ray absorption near edge spectroscopy study.Dalton Trans. 2010 Mar 28;39(12):3057-64. doi: 10.1039/b922557a. Epub 2010 Jan 28. Dalton Trans. 2010. PMID: 20221540 Free PMC article.
References
-
- Cammack R, Frey M, Robson R. Hydrogen as a Fuel: Learning from Nature. London: Taylor & Francis; 2001.
-
- Linden E, Burgdorf T, Bernhard M, Bleijlevens B, Friedrich B, Albracht S. J. Biol. Inorg. Chem. 2004;9:616–626. - PubMed
-
- Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. Chem. Rev. 2007;107:4273–4303. - PubMed
-
- Brecht M, van Gastel M, Buhrke T, Friedrich B, Lubitz W. J. Am. Chem. Soc. 2003;125:13075–13083. - PubMed
-
- Fehlhammer WP, Fritz M. Chem. Rev. 1993;93:1243–1280.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

