Coordination chemistry of [HFe(CN)(2)(CO)(3)](-) and its derivatives: toward a model for the iron subsite of the [NiFe]-hydrogenases
- PMID: 19374433
- PMCID: PMC2732431
- DOI: 10.1021/ic900200s
Coordination chemistry of [HFe(CN)(2)(CO)(3)](-) and its derivatives: toward a model for the iron subsite of the [NiFe]-hydrogenases
Abstract
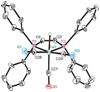
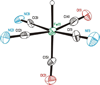
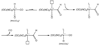
The photoreaction of Fe(CO)(5) and cyanide salts in MeCN solution affords the dianion [Fe(CN)(2)(CO)(3)](2-), conveniently isolated as [K(18-crown-6)](2)[Fe(CN)(2)(CO)(3)]. Solutions of [Fe(CN)(2)(CO)(3)](2-) oxidize irreversibly at -600 mV (vs Ag/AgCl) to give primarily [Fe(CN)(3)(CO)(3)](-). Protonation of the dianion affords the hydride [K(18-crown-6)][HFe(CN)(2)(CO)(3)] with a pK(a) approximately 17 (MeCN). The ferrous hydride exhibits enhanced electrophilicity vs its dianionic precursor, which resists substitution. Treatment of [K(18-crown-6)][Fe(CN)(2)(CO)(3)] with tertiary phosphines and phosphites gives isomeric mixtures of [HFe(CN)(2)(CO)(2)L](-) (L = P(OPh)(3) and PPh(3)). Carbonyl substitution on [1H(CO)(2)](-) by P(OPh)(3) is first-order in both the phosphite and iron (k = 0.18 M(-1) s(-1) at 22 degrees C) with DeltaH(double dagger) = 51.6 kJ mol(-1) and DeltaS(double dagger) = -83.0 J K(-1) mol(-1). These ligands are displaced under an atmosphere of CO. With cis-Ph(2)PCH=CHPPh(2) (dppv), we obtained the monocarbonyl, [HFe(CN)(2)(CO)(dppv)](-), a highly basic hydride (pK(a) > 23.3) that rearranges in solution to a single isomer. Treatment of [K(18-crown-6)][HFe(CN)(2)(CO)(3)] with Et(4)NCN resulted in rapid deprotonation to give [Fe(CN)(2)(CO)(3)](2-) and HCN. The tricyano hydride [HFe(CN)(3)(CO)(2)](2-) is prepared by the reaction of [HFe(CN)(2)(CO)(2)(PPh(3))](-) and [K(18-crown-6)]CN. Similar to the phosphine and phosphite derivatives, [HFe(CN)(3)(CO)(2)](2-) exists as a mixture of all three possible isomers. Protonation of the hydrides [HFe(CN)(2)(CO)(dppv)](-) and [HFe(CN)(3)(CO)(2)](-) in acetonitrile solutions releases H(2) and gives the corresponding acetonitrile complexes [K(18-crown-6)][Fe(CN)(3)(NCMe)(CO)(2)] and Fe(CN)(2)(NCMe)(CO)(dppv). Alkylation of [K(18-crown-6)](2)[Fe(CN)(2)(CO)(3)] with MeOTf gives the thermally unstable [MeFe(CN)(2)(CO)(3)](-), which was characterized spectroscopically at -40 degrees C. Reaction of dppv with [MeFe(CN)(2)(CO)(3)](-) gives the acetyl complex, [Fe(CN)(2)(COMe)(CO)(dppv)](-). Whereas [Fe(CN)(2)(CO)(3)](2-) undergoes protonation and methylation at Fe, acid chlorides give the iron(0) N-acylisocyanides [Fe(CN)(CO)(3)(CNCOR)](-) (R = Ph, CH(3)). The solid state structures of [K(18-crown-6)][HFe(CN)(2)(CO)(dppv)], Fe(CN)(2)(NCMe)(CO)(dppv), and [K(18-crown-6)](2)[HFe(CN)(3)(CO)(2)] were confirmed crystallographically. In all three cases, the cyanide ligands are cis to the hydride or acetonitrile ligands.
Figures


References
-
- Cammack R, Frey M, Robson R. Hydrogen as a Fuel: Learning from Nature. London: Taylor & Francis; 2001.
-
- Linden E, Burgdorf T, Bernhard M, Bleijlevens B, Friedrich B, Albracht S. J. Biol. Inorg. Chem. 2004;9:616–626. - PubMed
-
- Fontecilla-Camps JC, Volbeda A, Cavazza C, Nicolet Y. Chem. Rev. 2007;107:4273–4303. - PubMed
-
- Brecht M, van Gastel M, Buhrke T, Friedrich B, Lubitz W. J. Am. Chem. Soc. 2003;125:13075–13083. - PubMed
-
- Fehlhammer WP, Fritz M. Chem. Rev. 1993;93:1243–1280.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

