Utility of hyaluronan oligomers and transforming growth factor-beta1 factors for elastic matrix regeneration by aneurysmal rat aortic smooth muscle cells
- PMID: 19374489
- PMCID: PMC2792063
- DOI: 10.1089/ten.tea.2008.0593
Utility of hyaluronan oligomers and transforming growth factor-beta1 factors for elastic matrix regeneration by aneurysmal rat aortic smooth muscle cells
Abstract
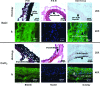
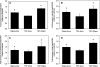
The progression of aortic aneurysms (AAs) is typically associated with an activated smooth muscle cell (SMC) phenotype, diminished density of mature medial elastic fibers, and an elevated presence of matrix-degrading enzymes, which ultimately leads to vessel rupture. Currently, no surgical or nonsurgical methods are available to regress aneurysms via regeneration of new elastic matrices, particularly because of inherently poor elastin synthesis by adult vascular cells and absence of tools to stimulate the same. We seek to address this void in this study. We recently showed 0.2 microg/mL of hyaluronan oligomers and 1 ng/mL of transforming growth factor-beta1 (termed elastogenic factors) to dramatically enhance elastin synthesis and matrix formation by healthy aortic SMCs. In this study, the effect of these factors, alone or together, on suppressing procalcific and elastolytic activities of aneurysmal vascular cells, and improving their elastin matrix synthesis and assembly is examined. Periadventitial injury with calcium chloride was used to induce AAs in rats, and approximately 45% increase in aortic diameter was observed after 4 weeks. Aneurysmal SMCs isolated from these AA segments produced higher levels of inflammatory markers matrix metalloproteinases-2 and 9 elastase activity and calcific deposits, while synthesizing significantly less collagen, tropoelastin, and matrix elastin proteins over a 3-week culture period, relative to healthy SMCs. While hyaluronan oligomers alone significantly suppressed aneurysmal cell proliferation and promoted 20-50% increases in collagen and elastin synthesis (p < 0.01), transforming growth factor-beta1 alone had no effect on cellular proliferation and elastin synthesis. However, provision of factors together resulted in significantly higher amounts of collagen/elastin protein synthesis and crosslinking, by upregulating lysyl oxidase and desmosine. Compared to their individual contributions, the factors together were highly effective in minimizing the release of inflammatory enzymes, and encouraging elastic fiber formation. Since elastic matrix amounts were one order of magnitude lower than that observed with healthy cells, even upon elastogenic stimulation at doses optimized previously for healthy cells, increased doses are likely required and must be reoptimized for diseased cells. Despite this, the results point to the potential utility of these elastogenic factors in regenerating elastic matrices within AAs.
Figures

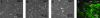
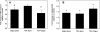
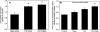
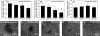


Similar articles
-
Evaluating smooth muscle cells from CaCl2-induced rat aortal expansions as a surrogate culture model for study of elastogenic induction of human aneurysmal cells.Tissue Eng Part A. 2011 Aug;17(15-16):1945-58. doi: 10.1089/ten.TEA.2010.0475. Epub 2011 Jun 14. Tissue Eng Part A. 2011. PMID: 21417692 Free PMC article.
-
Elastogenic inductability of smooth muscle cells from a rat model of late stage abdominal aortic aneurysms.Tissue Eng Part A. 2011 Jul;17(13-14):1699-711. doi: 10.1089/ten.TEA.2010.0526. Epub 2011 May 9. Tissue Eng Part A. 2011. PMID: 21341992 Free PMC article.
-
Transforming growth factor beta 1 and hyaluronan oligomers synergistically enhance elastin matrix regeneration by vascular smooth muscle cells.Tissue Eng Part A. 2009 Mar;15(3):501-11. doi: 10.1089/ten.tea.2008.0040. Tissue Eng Part A. 2009. PMID: 18847364 Free PMC article.
-
Atherosclerosis and matrix dystrophy.J Atheroscler Thromb. 2004;11(5):236-45. doi: 10.5551/jat.11.236. J Atheroscler Thromb. 2004. PMID: 15557705 Review.
-
Tissue engineering and regenerative strategies to replicate biocomplexity of vascular elastic matrix assembly.Tissue Eng Part B Rev. 2012 Jun;18(3):203-17. doi: 10.1089/ten.TEB.2011.0521. Epub 2012 Mar 2. Tissue Eng Part B Rev. 2012. PMID: 22224468 Free PMC article. Review.
Cited by
-
Advances in biomimetic regeneration of elastic matrix structures.Drug Deliv Transl Res. 2012 Oct;2(5):323-50. doi: 10.1007/s13346-012-0070-6. Drug Deliv Transl Res. 2012. PMID: 23355960 Free PMC article.
-
Evaluating smooth muscle cells from CaCl2-induced rat aortal expansions as a surrogate culture model for study of elastogenic induction of human aneurysmal cells.Tissue Eng Part A. 2011 Aug;17(15-16):1945-58. doi: 10.1089/ten.TEA.2010.0475. Epub 2011 Jun 14. Tissue Eng Part A. 2011. PMID: 21417692 Free PMC article.
-
Elastogenic inductability of smooth muscle cells from a rat model of late stage abdominal aortic aneurysms.Tissue Eng Part A. 2011 Jul;17(13-14):1699-711. doi: 10.1089/ten.TEA.2010.0526. Epub 2011 May 9. Tissue Eng Part A. 2011. PMID: 21341992 Free PMC article.
-
Abdominal Aortic Aneurysm: Evolving Controversies and Uncertainties.Int J Angiol. 2018 Jun;27(2):58-80. doi: 10.1055/s-0038-1657771. Epub 2018 May 29. Int J Angiol. 2018. PMID: 29896039 Free PMC article. Review.
-
Deletion of type VIII collagen reduces blood pressure, increases carotid artery functional distensibility and promotes elastin deposition.Matrix Biol Plus. 2021 Sep 29;12:100085. doi: 10.1016/j.mbplus.2021.100085. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34693248 Free PMC article.
References
-
- Milewicz D.M. Dietz H.C. Miller D.C. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111:e150. - PubMed
-
- Daugherty A. Cassis L.A. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429. - PubMed
-
- Rentschler M. Baxter B.T. Pharmacological approaches to prevent abdominal aortic aneurysm enlargement and rupture. Ann NY Acad Sci. 2006;1085:39. - PubMed
-
- Mecham R.P. Broekelmann T.J. Fliszar C.J. Shapiro S.D. Welgus H.G. Senior R.M. Elastin degradation by matrix metalloproteinases. Cleavage site specificity and mechanisms of elastolysis. J Biol Chem. 1997;272:18071. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

