Potential roles of Arnt2 in zebrafish larval development
- PMID: 19374551
- PMCID: PMC3140857
- DOI: 10.1089/zeb.2008.0536
Potential roles of Arnt2 in zebrafish larval development
Abstract
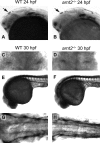
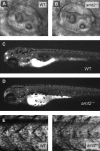
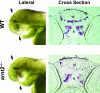
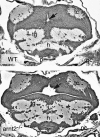
The aryl hydrocarbon receptor nuclear translocator (ARNT) is a basic helix-loop-helix-PAS heterodimeric transcription factor that dimerizes with other basic helix-loop-helix-PAS proteins to mediate biological responses. The function of ARNT2 is poorly understood. Here we provide an initial characterization of the zebrafish arnt2 null (arnt2(-/-)) mutant to identify functions of Arnt2 during development. Arnt2(-/-) mutant zebrafish develop normally until 120 hours postfertilization (hpf ) when morphological changes and functional deficits occur. The C-start escape response initiated by either touch or startle stimuli is absent in the mutants. Brain ventricle size is markedly increased at 120 hpf. Heart ventricles are enlarged, with decreased ventricle wall thickness. A cardiac arrhythmia, characterized by missing beats, is also observed in the mutants. This is associated with bradycardia in arnt2(-/-) larvae. Dilated liver sinusoids merge abnormally to form an extensive, labyrinth-like network of vascular channels. External appearance of arnt2(-/-) larvae at 120 hpf is indistinguishable from wild type except that the swim bladder is not inflated. The arnt2(-/-) mutants are not debilitated when phenotypic effects are first detected at 120 hpf that culminate in mortality, 4 days later around 216 hpf. Gross morphological assessment of the development of forebrain, midbrain, and hindbrain regions, neuromasts and Mauthner neurons, inner ear semicircular canals and otoliths, primary motor neurons, trigeminal ganglia, and trunk skeletal muscles, before or when the arnt2(-/-) phenotype was observed, failed to demonstrate a difference from wild type. The only effect in arnt2(-/-) larvae that occurred before 120 hpf was a decrease in expression of sim1, an Arnt2 dimerization partner, in the hypothalamus and ventral thalamus at 72 hpf. Further research is needed to determine if the primary functions of Arnt2 occur during the larval stage, when the phenotype is observed, or earlier in development.
Figures




Similar articles
-
Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages.Development. 2009 Mar;136(6):1007-17. doi: 10.1242/dev.033878. Development. 2009. PMID: 19234064
-
Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure.Toxicol Sci. 2002 Aug;68(2):403-19. doi: 10.1093/toxsci/68.2.403. Toxicol Sci. 2002. PMID: 12151636
-
Reciprocal regulation of the basic helix-loop-helix/Per-Arnt-Sim partner proteins, Arnt and Arnt2, during neuronal differentiation.Nucleic Acids Res. 2013 Jun;41(11):5626-38. doi: 10.1093/nar/gkt206. Epub 2013 Apr 17. Nucleic Acids Res. 2013. PMID: 23599003 Free PMC article.
-
Understanding dioxin developmental toxicity using the zebrafish model.Birth Defects Res A Clin Mol Teratol. 2006 Jan;76(1):7-18. doi: 10.1002/bdra.20216. Birth Defects Res A Clin Mol Teratol. 2006. PMID: 16333842 Review.
-
The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces.Curr Mol Med. 2013 Aug;13(7):1047-65. doi: 10.2174/15665240113139990042. Curr Mol Med. 2013. PMID: 23116263 Review.
Cited by
-
Bidirectional crosstalk between Hypoxia-Inducible Factor and glucocorticoid signalling in zebrafish larvae.PLoS Genet. 2020 May 7;16(5):e1008757. doi: 10.1371/journal.pgen.1008757. eCollection 2020 May. PLoS Genet. 2020. PMID: 32379754 Free PMC article.
-
Analysis of postembryonic heart development and maturation in the zebrafish, Danio rerio.Dev Dyn. 2012 Dec;241(12):1993-2004. doi: 10.1002/dvdy.23882. Epub 2012 Nov 5. Dev Dyn. 2012. PMID: 23074141 Free PMC article.
-
Fumonisin B1 Production by Fusarium Species and Mycotoxigenic Effect on Larval Zebrafish.Trop Life Sci Res. 2020 Oct;31(3):91-107. doi: 10.21315/tlsr2020.31.3.7. Epub 2020 Oct 15. Trop Life Sci Res. 2020. PMID: 33214858 Free PMC article.
-
ARNT2 Regulates Tumoral Growth in Oral Squamous Cell Carcinoma.J Cancer. 2016 Mar 26;7(6):702-10. doi: 10.7150/jca.14208. eCollection 2016. J Cancer. 2016. PMID: 27076852 Free PMC article.
-
Homeostatic Regulation of Glucocorticoid Receptor Activity by Hypoxia-Inducible Factor 1: From Physiology to Clinic.Cells. 2021 Dec 7;10(12):3441. doi: 10.3390/cells10123441. Cells. 2021. PMID: 34943949 Free PMC article. Review.
References
-
- Sogawa K. Numayama-Tsuruta K. Takahashi T. Matsushita N. Miura C. Nikawa J, et al. A novel induction mechanism of the rat CYP1A2 gene mediated by Ah receptor-Arnt heterodimer. Biochem Biophys Res Commun. 2004;318:746–755. - PubMed
-
- Rowlands JC. Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

