The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus
- PMID: 19374556
- PMCID: PMC2799113
- DOI: 10.1086/598967
The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus
Abstract
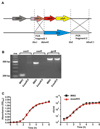
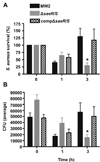
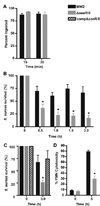
Methicillin-resistant Staphylococcus aureus is problematic both in hospitals and in the community. Currently, we have limited understanding of mechanisms of innate immune evasion used by S. aureus. To that end, we created an isogenic deletion mutant in strain MW2 (USA400) of the saeR/S 2-component gene regulatory system and studied its role in mouse models of pathogenesis and during human neutrophil interaction. In this study, we demonstrate that saeR/S plays a distinct role in S. aureus pathogenesis and is vital for virulence of MW2 in a mouse model of sepsis. Moreover, deletion of saeR/S significantly impaired survival of MW2 in human blood and after neutrophil phagocytosis. Microarray analysis revealed that SaeR/S of MW2 influences expression of a wide variety of genes with diverse biological functions. These data provide new insight into how virulence is regulated in S. aureus and associates a specific staphylococcal gene-regulatory system with invasive staphylococcal disease.
Conflict of interest statement
Figures


References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998 Aug 20;339(8):520–532. - PubMed
-
- Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus-Minnesota and North Dakota, 1997 – 1999. JAMA. 1999;(282):1123. - PubMed
-
- Adem PV, Montgomery CP, Husain AN, et al. Staphylococcus aureus sepsis and the Waterhouse-Friderichsen syndrome in children. N Engl J Med. 2005 Sep 22;353(12):1245–1251. - PubMed
-
- Gillet Y, Issartel B, Vanhems P, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002 Mar 2;359(9308):753–759. - PubMed
-
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007 Oct 17;298(15):1763–1771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

